1. Background
Viral hepatitis is among the most prevalent and vital diseases contributing to high global mortality and disability (1). The 2024 global hepatitis report from the World Health Organization (WHO) indicates a rise in fatalities attributed to viral hepatitis. The disease leads to 1.3 million deaths each year, ranking it as the second most lethal infection worldwide, similar to tuberculosis (2).
Currently, there are five main types of viral hepatitis (3). The majority of the global burden of viral hepatitis is induced by the hepatitis A, B, C, D, and E viruses (3, 4). The primary viral hepatitis types are A, B, and C (5). Another type, hepatitis E, was first identified in 1978 during an epidemic in the Kashmir Valley of India, where 52,000 individuals were affected, leading to the death of 1,700 people (6).
Hepatitis A is an infectious disease caused by the hepatitis A virus (HAV), which belongs to the Picornaviridae family and especially occurs in countries characterized by low socioeconomic status and inadequate sanitation (7). Hepatitis E virus (HEV), a member of the family Hepeviridae (8), leads to a viral disease similar to HAV (9). The causative agents of these two viral diseases lack an envelope and have a positive-sense single-stranded RNA (ssRNA) genome (10-12). The HAV and HEV both cause acute liver diseases and are typically transmitted through the fecal-oral route via contaminated food and water, as well as person-to-person contact (3, 9, 13, 14). Following an incubation period of approximately 28 days (15 - 50 days) for HAV and 40 days (15 - 60 days) for HEV, these viruses can lead to either clinical illness or remain asymptomatic in individuals (7, 15, 16). Hepatitis A continues to be self-limiting and does not develop into a chronic liver condition. The acute phase of the disease is characterized by the presence of hepatitis A-specific IgM antibodies in the serum (17). A more recent nationwide study on the seroprevalence rate of HAV in the Iranian population indicated that out of 5,419 participants from 12 provinces of Iran, 3,603 (66.5%) of people were seropositive for HAV-immunoglobulin G (IgG), which shows that vaccination against HAV is needed, at least for high-risk people (18). Additionally, the predicted outbreak of HEV in Iran is around 10%, which is noteworthy due to the virus's characteristics (19). While a vaccine for HAV is already available and has led to a significant reduction in the prevalence of the disease, several vaccines for HEV are currently being developed, with some already available in China, showing promising results (20). The HEV is the only zoonotic virus among the different types of hepatitis viruses. It is shown that there are abundant animal reservoirs for HEV, including pigs, chickens, rabbits, sheep, and others, which makes HEV a unique hepatitis infection (21). Pregnant women are the highest-risk group for HEV infection, with a mortality rate of up to 25% reported in this population (13, 20, 22). Furthermore, the mortality rate rises in pregnant cases of fulminant hepatitis (23). However, hepatitis A infection is rarely reported in pregnancy compared to HEV and shows a low mortality rate of 0.1 - 2.1% (13, 20). Conversely, both have similar mortality rates (0.1 - 4%) among the general population (13, 20). Therefore, HAV and HEV pose special problems to public hygiene (20). Unfortunately, few studies in Iran report the seroepidemiology of these two viruses in the general population. Moreover, Ardabil, a province in the northwest of Iran, has a high incidence of gastrointestinal diseases such as gastric cancer due to various factors contaminating the digestive system (24), and the seroprevalence of hepatitis A and E, as intestinal hepatitis infections, is undetermined in this region.
2. Objectives
Since there are no prior studies relevant to the seroprevalence measurement of anti-HEV and antibodies to HAV (anti-HAV) antibodies in Ardabil, this study was conducted to investigate the seroprevalence of these viruses and some associated risk factors. The results of such research could prove effective in future health programs for Ardabil and contribute to predicting the probability of potential hepatitis epidemics.
3. Methods
3.1. Study Design and Sample Size
This descriptive population-based cross-sectional study was conducted between 2018 and 2019 at the Digestive Disease Research Center of Ardabil University of Medical Sciences. Serum samples were taken from individuals who were undergoing the epidemiological investigation of gastric malignancies (ENIGMA) study. The samples were randomly selected from different areas of Ardabil city. The ENIGMA study consists of a series of international prevalence surveys in high and low gastric cancer risk areas: Prevalence surveys of Helicobacter pylori infection (ENIGMA 1); and prevalence studies of gastric histological changes (ENIGMA 2)]. The ENIGMA I includes age-stratified random samples of subjects aged 1 - 69 and investigates age-specific H. pylori prevalence and cofactors (bacterial, host, and environmental) that could explain the regional differences, in addition to predicting future rates of gastric cancer and assessing antibiotic resistance in each area.
The study protocol received ethical approval from the Ethics Committee of the Students Research Committee (IR.ARUMS.REC.1403.104), Faculty of Medicine, Ardabil University of Medical Sciences. Seven hundred individuals were included in the study. The sample size was determined by a statistician based on the expected seroprevalence rates of HAV and HEV, aiming for a precision of ± 5% with a 95% confidence interval (CI). To enhance the representativeness of the sample, participants were stratified by age and gender. The study included individuals aged 1 to 69 years old who were permanent residents of Ardabil city. They were categorized into seven ten-year age groups, with each group comprising 50 men and 50 women (100 participants per group).
Inclusion criteria: (A) be part of the ENIGMA study; (B) individuals aged 1 to 69 years old; (C) permanent residents of Ardabil; (D) voluntary provision of written informed consent (for adults) or written informed consent obtained from parents/legal guardians (for children under 18 years of age). Exclusion criteria: (A) individuals over 69 years old; (B) patients with known chronic debilitating diseases (e.g., chronic liver disease of non-viral etiology, immunosuppression, or other conditions that might alter antibody response or confound serological interpretation); (C) individuals who declined to participate or were unable to provide informed consent; (D) patients with insufficient blood sample volume for serological analysis.
3.2. Data Collection Method
Five milliliters of venous blood were obtained from all participants, and the sera were separated using a centrifuge and stored in a freezer at -80°C until examination. The samples were tested separately for total anti-HAV and HEV antibodies. A checklist was also completed for each individual, containing questions about demographic characteristics, environmental factors, and some determined risk factors for infections.
3.3. Laboratory Testing
Serological testing for anti-HAV and HEV antibodies was performed using an enzyme-linked immunosorbent assay (ELISA) kit (HAV IgG ELISA kit, antibody diagnostic kit for HAV, BIOVANTION, catalog No: BE302A, China), according to the test manual. This kit employs a solid-phase, indirect ELISA method for the detection of IgG anti-HAV in serum or plasma with a two-step incubation procedure. The polystyrene microwell is pre-coated with purified natural HAV antigen. The HRP-conjugated (horseradish peroxidase) mouse anti-human IgG (γ chain) monoclonal antibody serves as a tracer. TMB (3, 3', 5, 5'-tetramethylbenzidine) is a substrate for HRP. The enzyme reaction with substrate TMB produces a color change, and the intensity of the absorbance at 450 nm indicates the presence or absence of anti-HAV antibodies IgG in the sample. The test was specific, sensitive, reproducible, and easy to operate. It reveals a blood screen for HAV infection.
The exact test procedure was as follows: (1) All kit reagents and specimens were brought to room temperature before use (approximately 30 minutes); (2) for each test, one blank, two positive, and three negative controls were set. One hundred μL of positive and negative control serum were added into their respective wells; (3) 50 μL of serum was added to each of the test wells, and the sample wells were mixed by pipetting up and down; (4) the wells were covered with seal paper and incubated for 30 minutes at 37°C; (5) the liquid of all wells was discarded, and the wells were filled with wash solution. After 15 seconds, the process was repeated. The wells were washed 5 times and dried after the last wash; (6) 100 μL of enzyme conjugate was added to each well except the blank; (7) the wells were covered with seal paper and incubated for 30 minutes at 37°C; (8) step 6 was repeated; (9) 50 μL of substrate A and B were added respectively to each well, including the blank well. Then, the wells were mixed gently, protected from light, and incubated for 15 minutes at 37°C; (10) 50 μL of stop solution was added into each well to stop the reaction, including the blank well; (11) the absorbance was measured at 450 nm.
3.4. Statistical Analysis
The data collected were analyzed using SPSS software v24 and presented descriptively within frequency distribution tables. Descriptive statistics were used to summarize the numerical variables, including mean, standard deviation, and median. Considering the different ages of the studied individuals, the most important goal of the study was to examine the exposure trend at different ages. Categorical variables were displayed using tables with percentages, ensuring a thorough understanding of the data. The chi-square test was employed to assess the relationship between categorical variables and the prevalence of the viruses. Furthermore, the t-tests and Mann-Whitney U test were utilized to compare the means of numerical variables between different groups. The t-test was applied for data that followed a normal distribution, while the Mann-Whitney U test was used for non-normally distributed data. Both tests aimed to identify significant differences in the means of the variables between groups. A significance level of 0.05 was set, meaning a P-value less than 0.05 was considered statistically significant.
4. Results
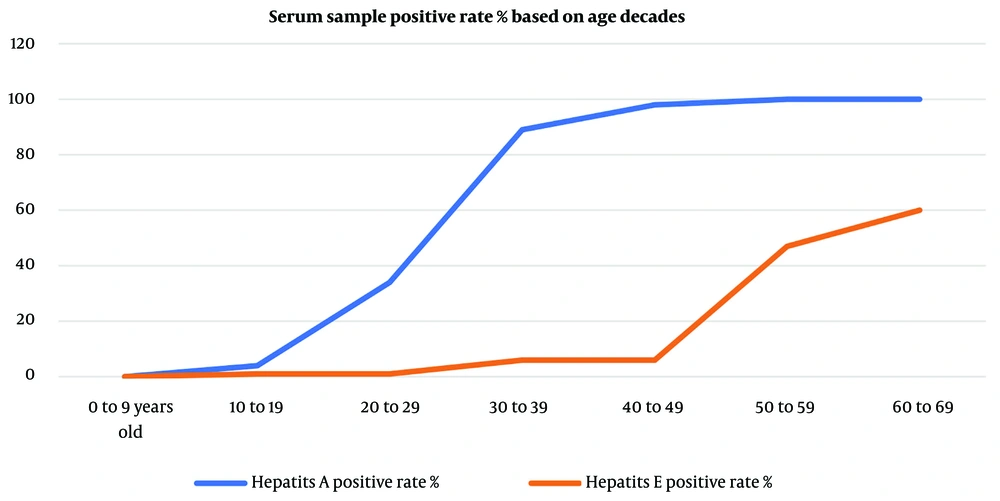
The overall seroprevalence of HAV IgG antibodies among the study participants was 60.3%, while the seroprevalence of HEV IgG antibodies was notably lower at 19.3% (Table 1). The seroprevalence of both HAV and HEV IgG antibodies demonstrated a different age-dependent rate (Table 2). This table also lists a few inconclusive results, where the test result was not clearly stated. The number of tests with equivocal results was small, and these results were considered negative in the statistical analysis. However, it is possible that the high number of equivocal test results in the over-60s in the hepatitis E group may have been due to patients with a history of actual infection who experienced a decline in serum antibody concentrations as a result of increasing age.
| Virus | Seropositive | Male | Female |
|---|---|---|---|
| HAV | 422 (60.3) | 355 (50.7) | 345 (49.3) |
| HEV | 135 (19.3) | 352 (50.3) | 348 (49.7) |
Overall Seroprevalence of Hepatitis A Virus and Hepatitis E Virus Immunoglobulin G Antibodies in Ardabil (N = 700) a
| Age Group (y) | HAV Seropositive | Hepatitis A Equivocal Results Rate | HEV Seropositive | Hepatitis E Equivocal Results Rate |
|---|---|---|---|---|
| 1 - 9 | 1 | 5 | 0 | 3 |
| 10 - 19 | 4 | 4 | 1 | 1 |
| 20 - 29 | 33 | 7 | 1 | 1 |
| 30 - 39 | 88 | 4 | 6 | 9 |
| 40 - 49 | 96 | 6 | 20 | 3 |
| 50 - 59 | 100 | 4 | 47 | 3 |
| 60 - 69 | 100 | 1 | 60 | 18 |
Seroprevalence of Hepatitis A Virus and Hepatitis E Virus Immunoglobulin G Antibodies by Age Group (Number = 100) a
In the group studied for hepatitis A, the effect of some variables in the questionnaire on the prevalence of hepatitis A was measured. There was no significant difference in gender, smoking, and alcohol consumption between the two groups of positive and negative serology. However, lower maternal literacy and the use of unsanitary water were significantly associated with an increase in serum positivity. Due to the small number of positive samples, the analysis of the above variables was not performed in the hepatitis E group.
The results show that there is a significant difference in the seroprevalence of hepatitis A and E. As Figure 1 shows, hepatitis A has a significant upward trend starting from the beginning of the third decade, and by the end of the fourth decade, almost 100% of the tested individuals had a history of hepatitis A. These results mean that hepatitis A was endemic in Ardabil twenty years ago, but in the last twenty years, the circulation of the virus has decreased significantly. This finding indicates that the circulation of the virus in the urban population of children and adolescents has decreased significantly compared to twenty years ago. On the other hand, hepatitis E shows a significant upward trend beginning in the early fifth decade, indicating that Ardabil experienced a hepatitis outbreak 40 to 50 years before the study was conducted. In the last forty years, only isolated cases of infected individuals have been found in the studied samples. This finding indicates that hepatitis E is not currently a common problem in the province, but due to the lack of herd immunity, there is a risk of outbreaks due to contamination of water or food sources.
5. Discussion
The HAV and HEV are two important enteric hepatotropic viruses worldwide that cause liver disease (20). Despite significant genetic differences, both viruses are transmitted through the fecal-oral route and have similar gastrointestinal symptoms, making them clinically unrecognizable (25). Water quality, sanitation coverage, food hygiene, and public health awareness could be contributing factors to HAV and HEV transmission (26). Therefore, in societies with low sanitation coverage, most people in childhood and adolescence are exposed to these viruses. On the other hand, the prevalence of such diseases during childhood is very low in industrialized countries. In these societies, young and middle-aged people are usually infected (27). This study represents the first comprehensive report on the seroprevalence of HAV and HEV by age in Ardabil, a city in the northwest of Iran, thereby filling a significant gap in the regional epidemiological data.
None of the viruses showed statistically significant differences in prevalence between genders. This suggests that both males and females are equally susceptible to these infections and that gender-specific behaviors may not play a major role in transmission.
In a similar prospective cross-sectional study conducted by Bawazir et al., the seroprevalence rates of HAV and HEV in Aden, Yemen, were described (26). In this study, 466 of 538 (86.6%) participants had anti-HAV IgG antibodies, and 38 of 356 (10.7%) participants tested had anti-HEV IgG antibodies. This study shows a significant difference in the seroprevalence between age groups for both HAV and HEV, similar to the results shown in our research. The seroprevalence rates of HAV and HEV increase significantly with aging. In other words, the highest prevalence rates reported by Bawazir et al. (26) were among adults aged ≥ 45 years, 99% for HAV, and nearly 20% for HEV. Similarly, our study showed that 99.3% of adults aged ≥ 40 are positive for HAV and 14.47% are HEV seropositive. The overall seroprevalence of HAV in Ardabil (60.30%) was lower than reported in Aden (86.6%). This could be due to better socioeconomic factors and sanitation coverage of people in Ardabil. However, a higher rate of HEV (19.30%) was reported in Ardabil than in Aden (10.7%). Also, in our study, neither HAV nor HEV showed statistically significant differences in prevalence between genders, similar to Aden's study. This suggests both males and females are equally vulnerable to these infections.
In another study conducted by Behzadi et al. about the seroprevalence of various viral hepatitis infections in the Hormozgan province of southern Iran (14), a higher anti-HAV antibody prevalence was reported compared with Ardabil. Of 562 participants in this study, 524 (93.2%) were positive for HAV. This result likely reflects broader socioeconomic factors, including living conditions, hygiene practices, and access to clean water in Ardabil. Behzadi et al. also showed that contaminated water sources due to possible untreated sewage of humans or improper disposal systems could be important reasons for such infection (14).
Our findings for Ardabil contribute significantly to the understanding of HAV and HEV epidemiology within Iran, allowing for crucial regional comparisons. The higher anti-HAV antibody prevalence reported in Hormozgan province (93.2%) (14) and Tehran province (90.0%) (28) are consistent with the results of testing at older ages in those two studies. Conversely, the seroprevalence of HEV in Ardabil (19.30%) was notably higher than that reported in Tehran (9.3%) (28). This disparity in HEV prevalence could be attributed to a greater degree of animal contact and livestock farming in Ardabil, given HEV's zoonotic nature, or potentially localized differences in sanitation and water treatment. These regional variations underscore the need for geographically tailored public health interventions.
Given the substantial proportion of the population already exposed, particularly older age groups, targeted vaccination campaigns, especially for HAV, should be considered to protect younger, susceptible cohorts and prevent future outbreaks. Furthermore, the identified associations with environmental factors, such as water sources and sanitation facilities, highlight critical areas for intervention. Improving access to clean water and enhancing sanitation infrastructure are paramount to disrupting fecal-oral transmission routes. Public health education initiatives focusing on personal hygiene practices, safe food handling, and awareness of transmission risks are also essential to reduce the burden of these infections in Ardabil. The higher HEV prevalence compared to Tehran also suggests a need for specific attention to animal-human transmission routes in Ardabil, potentially through veterinary public health programs and education for those in contact with livestock. The low prevalence of hepatitis A in the first and second decades of life of the subjects tested in the present study indicates that the incidence of the disease has decreased, possibly due to improved health literacy and environmental health indicators, and an overall decrease in the incidence of diseases transmitted through contaminated water and food. The number of tests with indeterminate or equivocal results in the 60 - 69 age group in patients with hepatitis E was 18 times higher than in patients with hepatitis A (18 cases versus one case, respectively). If we consider these test results to be positive, which have decreased due to the passage of time and the decrease in immune memory, we can conclude that due to the continuous circulation of the HAV in the community, the immune memory of the elderly remains active, while the immune memory for hepatitis E has weakened due to the lack of active circulation of the virus in the community.
5.1. Conclusions
Hepatitis A is moving away from being an endemic disease, and this may necessitate vaccination in children. Since hepatitis A has a much lower mortality rate in childhood compared to the post-puberty period, the decrease in virus circulation in the community and the increase in cases at older ages are alarming for the higher mortality rate of the disease in susceptible individuals in the coming years. This phenomenon emphasizes the need to investigate the necessity for a hepatitis A vaccine. Hepatitis E is not currently endemic in Ardabil city, and the prevalence of infection in individuals aged 1 to 40 years is very low. This finding indicates that residents of Ardabil are highly susceptible to hepatitis E disease and are at risk of outbreaks due to food or water contamination. The significant number of sera positive for anti-HEV IgG antibodies also indicates that hepatitis E should be included in the differential diagnosis of clinical hepatitis in cases where the test results for hepatitis A, B, and C are negative. The study underscores the persistent public health challenge posed by HAV and HEV infections in Ardabil, highlighting the continued necessity of prioritizing enhanced personal hygiene, improved water source quality, comprehensive sanitation coverage, and increased public health awareness.
5.2. Limitations
This study is a population-based cross-sectional study whose samples are truly representative of the population, but because the incidence of the disease over time has been strongly influenced by public health, its results should be interpreted according to the circumstances. Hepatitis A and E viruses have different reservoirs, and their circulation in the Ardabil ecosystem has been different. The study did not focus on investigating behavioral factors affecting the incidence of the disease, and this is one of its limitations.