1. Background
Toxoplasma gondii is considered one of the most prevalent parasitic infections worldwide, with approximately one-third of the global population being seropositive for the parasite. The infection has a global seroprevalence, with higher prevalence rates in tropical and subtropical areas (1, 2). Infection typically occurs through the ingestion of food or drinking water contaminated with oocysts, consumption of raw or undercooked meat containing tissue cysts, vertical transmission from mother to fetus, and less commonly, through blood transfusions or organ transplantation (3-6). The infection is typically asymptomatic in immunocompetent individuals, whereas in immunocompromised individuals, it can cause severe, progressive, and life-threatening complications (7). Patients with conditions such as aplastic anemia, sickle cell anemia, and thalassemia, who require frequent blood transfusions to survive, may experience irreparable complications from opportunistic infections such as T. gondii due to their immunodeficiency (5, 8). Recent evidence suggests that T. gondii can be transmitted via transfusion of whole blood and white blood cells, with the parasite capable of surviving for up to 50 days in blood stored at 5°C (9, 10). Asymptomatic blood donors in the acute phase of infection with parasitemia may play an important role in the transmission of this parasite (11-13). In Iran, the seroprevalence of T. gondii varies considerably across different geographical regions (14). In recent decades, transfusion-transmitted infections (TTIs), including T. gondii from blood donors, have become a global concern for blood recipients, particularly among immunocompromised populations (5).
2. Objectives
It is worth mentioning that screening for T. gondii before transfusion has not been widely implemented. Since there is no evidence regarding the epidemiological data showing the seroprevalence of this infection in donated blood in this region in past years, the present research aimed to examine the seroprevalence of IgG and IgM antibodies against T. gondii among apparently healthy blood donors in the cities of Abadan and Khorramshahr, located in Khuzestan province, Iran.
3. Methods
3.1. Study Area
Abadan and Khorramshahr are cities located in Khuzestan province in southwestern Iran. Both cities are situated on flat plains and experience a hot desert climate. There is a noticeable variation in air temperature between summer and winter, with summer temperatures exceeding 50°C and winter temperatures typically ranging from 16°C to 20°C. Annual dust storms occur in this area. The average annual humidity is 45%, and the region is generally considered humid, with humidity levels reaching 100%.
3.2. Population
This cross-sectional study included 345 healthy volunteer blood donors who referred to central Blood Transfusion Organizations (BTO) affiliated with Abadan University of Medical Sciences (AUMS) in Abadan and Khorramshahr between May and September 2022. Convenience sampling was used due to logistical constraints; future studies should prioritize powered calculations. Written informed consent was obtained and signed by all participants prior to enrollment. Blood donations were routinely screened for five pathogens, including human T-lymphotropic virus 1 and 2 (HTLV1, 2), Hepatitis C virus (HCV), Hepatitis B virus (HBV), human immunodeficiency virus (HIV), and Treponema pallidum, in accordance with BTO guidelines (15). The study followed three inclusion criteria to select participants: (1) Volunteers aged ≥ 18 years; (2) individuals who provided informed consent to participate in the investigation; and (3) donors whose blood tests were negative for HTLV1, HTLV2, HCV, HBsAg, HIV, and T. pallidum. A structured questionnaire was completed by each blood donor, consisting of demographic information and several risk factors associated with T. gondii infection.
3.3. Serology
Approximately 10 mL of venous blood was collected from each eligible participant. After collection, the blood samples were centrifuged at 3500 rpm for 5 minutes in anticoagulant-free tubes. The serum samples were then separated and stored at -20°C until further analysis. The serum samples were evaluated for specific IgG and IgM antibodies against T. gondii using Torch-IgG, IgM-Trinity (Biotech Company), following the manufacturer’s protocols. Samples with values less than 0.9, between 0.9 and 1.1, and above 1.1 were considered negative, borderline, and positive, respectively (16). Sensitivity and specificity for IgG were 90 - 100% and 95 - 100%, respectively. For IgM, these values were 80 - 100% and 80 - 100%, respectively. The results were recorded by an enzyme-linked immunosorbent assay (ELISA) reader based on optical density (OD) values, and the antibody concentrations were interpreted according to the OD thresholds provided by the manufacturer.
3.4. Statistical Analysis
The data were analyzed descriptively following the collection of laboratory results and questionnaire responses. Descriptive statistics, including frequency and percentage, were used. The chi-square test was applied using SPSS software version 19 (SPSS Inc., Chicago, IL, USA). Furthermore, univariate logistic regression analysis was performed to evaluate the probable association between the seroprevalence of T. gondii infection (based on IgG antibody) and related risk factors. The level of significance was set at P < 0.05.
4. Results
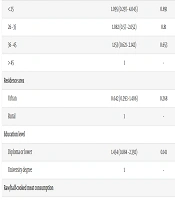
In this study, 97.1% of the blood donors were male. Among the participants, 31.9% were IgG positive, and 1.5% were positive for both IgM and IgG antibodies (Table 1). The majority of participants (42.02%) were aged between 36 and 45 years, and 91.9% lived in residential areas located in cities. Of the 345 participants, 6 individuals (1.8%) tested positive for IgM antibodies. Logistic regression analysis showed no significant association between the variables studied. Notably, 95.7% of participants reported not consuming raw or semi-cooked meat. Despite this, 31.5% of these individuals were IgG positive, and 1.5% were positive for both IgM and IgG antibodies; however, statistical analysis did not reveal a significant relationship between these cases (Table 2). Additionally, 78.3% of participants consumed properly washed vegetables, among whom 30.7% were IgG positive, with no significant association observed. Regarding contact with cats, 95.9% of cases had no contact with cats, yet 31.7% and 1.5% of these individuals were positive for IgG and both IgG/IgM antibodies, respectively. Among those who had contact with cats, no meaningful association was noticed with IgG positivity. Furthermore, 15.1% of participants had contact with soil, among whom 21 individuals (36.9%) were IgG positive. However, statistical analysis did not reveal any significant relationship. Among the participants, 115 individuals (33.3%) had blood type O positive, and 29.6% of these individuals were IgG positive. No significant relationship was observed between IgG positivity and blood type O positive. Despite statistical non-significance, the 42.9% IgG seroprevalence in rural donors (vs. 30.9% in urban donors) warrants further study due to potential environmental exposure differences.
| Variables | No. (%) | IgG Positive (%) | IgM Positive (%) | Both IgG/IgM Positive (%) |
|---|---|---|---|---|
| Gender | ||||
| Male | 335 (97.1) | 107 (31.9) | 6 (1.8) | 5 (1.5) |
| Female | 10 (2.9) | 3 (30) | - | - |
| Agegroups (y) | ||||
| < 25 | 12 (3.47) | 3 (25) | - | 1 (8.3) |
| 26 - 35 | 121 (35.07) | 39 (32.2) | 1 (1) | 1 (0.8) |
| 36 - 45 | 145 (42.02) | 48 (33.1) | 5 (3.1) | 2 (1.4) |
| > 45 | 67 (19.42) | 20 (29.9) | - | 1 (1.5) |
| Residencearea | ||||
| Urban | 317 (91.9) | 98 (30.9) | 6 (1.9) | 5 (1.6) |
| Rural | 28 (8.31( | 12 (42.9) | - | - |
| Educationlevel | ||||
| Diploma or lower | 237 (68.7) | 81 (34.2) | 4 (1.7) | 4 (1.7) |
| University degree | 108 (31.3) | 29 (26.9) | 2 (1.9) | 1 (0.9) |
| Raw/half-cookedmeatconsumption | ||||
| Yes | 15 (4.3) | 6 (40) | - | - |
| No | 330 (95.7) | 104 (31.5) | 6 (1.8) | 5 (1.5) |
| Eating properly washed vegetables | ||||
| Yes | 270 (78.3) | 83 (30.7) | 6 (2.2) | 3 (1.1) |
| No | 75 (21.7) | 27 (36) | - | 2 (2.7) |
| Contact with cat | ||||
| Yes | 14 (4.1) | 5 (35.7) | - | - |
| No | 331 (95.9) | 105 (31.7) | 6 (1.8) | 5 (1.5) |
| Contact with soil | ||||
| Yes | 52 (15.1) | 21 (39.6) | 1 (1.9) | - |
| No | 293 (84.9) | 89 (30.5) | 5 (1.7) | 5 (1.7) |
| Blood group and Rh | ||||
| A (Rh positive) | 90 (26.1) | 36 (40) | 1 (1.1) | 2 (2.2) |
| A (Rh negative) | 5 (1.4) | 3 (60) | - | - |
| B (Rh positive) | 85 (24.6) | 21 (24.7) | 2 (2.4) | 1 (1.2) |
| B (Rh negative) | 5 (1.4) | 1 (20) | - | - |
| AB (Rh positive) | 29 (8.4) | 9 (31) | - | 1 (3.4) |
| AB (Rh negative) | 2 (0.6) | 1 (50) | - | - |
| O (Rh positive) | 115 (33.3) | 34 (29.6) | 3 (2.6) | 1 (0.9) |
| O (Rh negative) | 14 (4.1) | 5 (35.7) | - | - |
Demographic Characteristics and Toxoplasma gondii Seroprevalence Among Healthy Blood Donors in Southwest Iran
| Variables | IgG Positive | |
|---|---|---|
| Crude OR (95% CI) | P-Value | |
| Gender | ||
| Male | 1.172 (0.297 - 4.618) | 0.821 |
| Female | 1 | - |
| Age groups (y) | ||
| < 25 | 1.095 (0.297 - 4.045) | 0.891 |
| 26 - 35 | 1.082 (0.57 - 2.052) | 0.81 |
| 36 - 45 | 1.153 (0.621 - 2.142) | 0.653 |
| > 45 | 1 | - |
| Residence area | ||
| Urban | 0.642 (0.293 - 1.406) | 0.268 |
| Rural | 1 | - |
| Education level | ||
| Diploma or lower | 1.454 (0.884 - 2.392) | 0.141 |
| University degree | 1 | - |
| Raw/half-cooked meat consumption | ||
| Yes | 1.352 (0.469 - 3.894) | 0.577 |
| No | 1 | - |
| Eating properly washed vegetables | ||
| Yes | 0.741 (0.436 - 1.26) | 0.269 |
| No | 1 | - |
| Contact with cat | ||
| Yes | 1.116 (0.365 - 3.41) | 0.847 |
| No | 1 | - |
| Contact with soil | ||
| Yes | 1.382 (0.757 - 2.526) | 0.292 |
| No | 1 | - |
| Blood group and Rh | ||
| A (Rh negative) | 3.429 (0.548 - 21.432) | 0.188 |
| A (Rh positive) | 1.67 (0.938 - 2.974) | 0.081 |
| B (Rh negative) | 0.571 (0.062 - 5.298) | 0.622 |
| B (Rh positive) | 0.798 (0.426 - 1.494) | 0.481 |
| AB (Rh negative) | 2.286 (0.139 - 37.592) | 0.563 |
| AB (Rh positive) | 1.203 (0.508 - 2.85) | 0.675 |
| O (Rh negative) | 1.27 (0.397 - 4.063) | 0.687 |
| O (Rh positive) | 1 | - |
Univariate (Crude OR) Logistic Regression Analysis of the Potential Risk Factors Associated with Toxoplasma gondii IgG Seroprevalence Among Healthy Blood Donors of Southwest Iran
5. Discussion
Given the necessity of reducing toxoplasmosis transmission and mitigating the complications of this infection, this cross-sectional study investigated the seroprevalence of T. gondii IgG and IgM antibodies among blood donors in Khorramshahr and Abadan, Iran, and examined associated epidemiological factors. Demographic characteristics and feeding behaviors were assessed alongside blood sampling for T. gondii. The main findings showed that the seroprevalence of T. gondii IgG antibodies among participants was 31.88% (110/345). This seroprevalence was higher than in previous studies conducted in regions such as Turkey (17), Mexico (18, 19), Chile (20), India (12), Malaysia (21), Mali (22), and Thailand (23), but aligns with global averages (5) and national estimates (14). Differences in T. gondii seroprevalence between studies may be attributed to geographic factors (5, 14). In a study aimed at evaluating the seroprevalence of infection in blood donors in China using a meta-analysis, 40 eligible studies were reviewed, reporting that among 49,784 blood donors in China, the prevalence of toxoplasmosis was 6.26% (24). The seroprevalence of T. gondii IgG antibody in Khorramshahr and Abadan was comparable to the global average (5). Statistical analysis of risk factors showed that the seroprevalence of T. gondii was not statistically different between men and women (P = 0.821), consistent with prior studies (25). Although blood donors with lower education levels exhibited a higher prevalence of toxoplasmosis, this association was not statistically significant. These findings could be attributed to differences in living conditions and increased likelihood of exposure to potential infection sources. Health education and promotion, especially avoiding eating raw and undercooked meat and avoiding contact with feline feces, are key recommendations to reduce the prevalence of toxoplasmosis (26). Consistent with other studies, the majority of participants in this study did not consume raw or semi-cooked meat, and no significant association was observed between this factor and T. gondii seropositivity (27). However, a study investigating T. gondii seroprevalence in blood donors in Boyar Ahmad city in Iran demonstrated that risk factors such as contact with soil and consumption of semi-cooked meat were significantly associated with T. gondii seropositivity (28). In fact, the consumption of semi-cooked meat, particularly lamb and goat meat containing tissue cysts, was known as an important source of T. gondii infection in that city (29, 30). Cultural dietary practices (e.g., thorough meat cooking) or underreported cat exposures may explain discrepancies. A hypothesis suggests that in Khorramshahr and Abadan, cities near the Persian Gulf, there is a dietary balance between the consumption of animal products and seafood. In contrast, in rural areas in northern Iran, the consumption of semi-cooked meat and unwashed vegetables are major risk factors for toxoplasmosis. Similarly, contact with cats and consuming raw vegetables and raw eggs have been recognized as risk factors for toxoplasmosis among blood donors in the southeast of Iran (30, 31). Among participants, 78.3% consumed properly washed vegetables, with 30.7% of this group testing IgG positive. However, no significant association was observed between vegetable consumption and T. gondii seropositivity. In a similar study, serological investigation and determination of the genotype of T. gondii among Iranian blood donors in Mazandaran province were conducted, and there was an association between the prevalence of T. gondii and sex, blood group, Rh, source of water, and meat of nymphs and contact with garden soil, contact with animals, but no significant relationship was found between drinking raw milk and consuming raw vegetables (32). This aligns with findings from other studies, which also did not identify a relationship between the consumption of vegetables and T. gondii infection (17, 33). Among the cases, 95.9% had no contact with cats. Among participants who had contact with cats (transient exposure), no meaningful association was observed between cat exposure and IgG seropositivity. Despite the fact that a large number of cases were not in contact with felines, other studies considered this variable as one of the main risk factors for T. gondii infection (34, 35). Cats are an important source of infection and can excrete large numbers of oocysts that can survive for months in humid environments. In addition, beetles and flies can spread oocysts from soil to food. Due to the amount of oocysts in the habitat, animals can be infected, which can cause an increase in infection among animals. Additionally, tissue cysts of infected rats may transmit to cats through hunting. Cats can pollute water, gardens, fields, and food by excreting large amounts of oocysts (32). Underreported cat exposures may explain discrepancies. In the current study, 15.1% of participants had contact with soil, with 36.9% of these individuals testing IgG positive. However, statistical analysis did not reveal a significant relationship between soil contact and seropositivity. A similar study aimed at serological investigation and determining the genotype of T. gondii in blood donors in Iran concluded that there was no meaningful association between the seroprevalence of T. gondii and garden soil (32). Among the participants, 33.3% had an O-positive blood group, with 29.6% testing IgG positive. Statistical analysis did not show a significant relationship between IgG positivity and the O-positive blood group. Similar studies have confirmed that there is no association between T. gondii IgG prevalence and ABO or Rhesus blood groups (36, 37). These results show that blood group is not a risk factor for infection (38, 39). It seems that the ABO/Rh non-association with T. gondii infection (Table 2) aligns with global literature, reinforcing blood groups’ irrelevance to T. gondii susceptibility.
5.1. Conclusions
The seroprevalence of T. gondii antibodies among blood donors in Khorramshahr and Abadan was estimated to be 31.88%. Although seroprevalence does not indicate active T. gondii infection, there remains a risk of toxoplasmosis transmission via blood transfusions. Given that most blood recipients are at-risk patients, it is crucial to incorporate screening for T. gondii antibodies into the routine protocols of Iran’s Blood Transfusion Organization, particularly in the cities of Khorramshahr and Abadan.
5.2. Limitations
In this study, only the ELISA serologic test was performed, which has high sensitivity (90 - 100% for IgG and 80 - 100% for IgM) and specificity (95 - 100% for IgG and 80 - 100% for IgM). The absence of molecular tests to confirm serological results, which could have enhanced diagnostic accuracy, is the main limitation of this research project.