1. Background
Pre-eclampsia (PE) is a multisystem disorder of unknown etiology, characterized by the development of hypertension — defined as blood pressure of 140/90 mmHg or higher — accompanied by edema, proteinuria, or both, induced by pregnancy after the 20th week (1). Gestational hypertension (GH) is often regarded as a transitional condition, with the majority of women who develop the disorder between 24 and 35 weeks of gestation progressing to PE (2). Pre-eclampsia and GH are commonly categorized as hypertensive disorders complicating pregnancy. It has been suggested that an imbalance in angiogenic markers and increased oxidative stress are key contributing factors to the endothelial dysfunction observed in both PE and GH (3).
Magnesium deficiency impairs angiogenesis by inhibiting endothelial cell growth and migration (4). Inadequate nitric oxide (NO)-mediated vasodilation leads to vasoconstriction of the feto-placental vessels, resulting in insufficient perfusion in severe PE cases (5). Nitric oxide is crucial for maintaining smooth muscle tone, and magnesium is known to influence NO levels (6). Magnesium is essential for endothelial cell proliferation, while NO is a well-established potent vasodilator. Placental hypoxia and reduced NO levels contribute to oxidative stress. Measuring NO levels during the first and second trimesters of pregnancy may provide insight into hypertension-associated changes.
2. Objectives
The present study aimed to identify the serum levels of magnesium and NO during the first and second trimesters of pregnancy, in order to analyze their differences in PE and GH, and to predict the time of onset of PE/GH using serum magnesium and NO levels.
3. Methods
3.1. Study Design and Participants
This cohort study was conducted over a period of three and a half years in the Department of Biochemistry, Kasturba Medical College, Manipal, and the Department of Obstetrics and Gynaecology, Dr. TMA Pai Hospital, Udupi. The project proposal, patient information sheet, and consent form were approved by the Institutional Ethics Committee of Kasturba Medical College (IEC 290/2013), dated 10-07-2013.
The sample size was statistically calculated in a 1:2 case-to-control ratio, and a total of 900 pregnant women were initially recruited into the cohort. Blood samples and detailed proformas were obtained from all 900 participants. However, only a subset of segregated cases and controls in a 1:3 ratio was used for biochemical analysis. Serum magnesium was assayed using a colorimetric quantitative assay kit from Coral Clinical Systems, India, and serum NO was assayed using the Griess method from AAT Bioquest, USA.
3.2. Eligibility Criteria
Two sets of inclusion and exclusion criteria had to be fulfilled for participation in the study. Inclusion criteria were: (1) Pregnant women aged 18 - 45 years; (2) attendees of the outpatient department at Dr. TMA Pai Hospital, Udupi; (3) ultrasound-confirmed single live pregnancy at ≤ 12 weeks of gestation; (4) recruitment period: August 2013 to August 2016. Cases were pregnant women with BP ≥ 140/90 mmHg in the left lateral position and/or presence of 1+, 2+, or 3+ urine protein by dipstick at any time after 20 weeks of gestation but before delivery. Controls were age-matched healthy pregnant women with BP ≤ 140/90 mmHg and no 1+, 2+, or 3+ proteinuria by dipstick. Exclusion criteria included: (1) Presentation after the first trimester; (2) multiple pregnancies; (3) assisted reproduction pregnancies; (4) pre-existing hypertension or diabetes; (5) chronic illnesses such as HIV, tuberculosis, rheumatoid arthritis, or chronic kidney disease; (6) current pregnancy abortion; (7) gestational diabetes onset at or before 20 weeks; and (8) chronic hypertension.
Participants meeting all inclusion and none of the exclusion criteria, attending in both the first trimester (up to 12+ weeks) and second trimester (13 - 28 weeks), were followed through to delivery. Based on pregnancy outcomes, they were classified into: (A) controls: Healthy pregnancies and (B) cases: Pregnancies developing GH/PE.
Upon obtaining informed consent, 4 mL of random blood was collected from participants during the first trimester (≤ 12+ weeks) and again during the second trimester (16 - 20 weeks), into plain vacutainers. The samples were allowed to clot and transported on ice to the Department of Biochemistry, Kasturba Medical College, Manipal. Samples were centrifuged at 5000 rpm for 5 minutes, and the serum was aliquoted and stored at -80°C for analysis. A detailed patient proforma was completed for each participant, including comprehensive information about the current pregnancy.
4. Results
Table 1 depicts the results of the comparison of serum magnesium and NO levels between healthy controls and PE/GH cases using the Mann-Whitney test. Serum magnesium levels were found to be higher in the first trimester and lower in the second trimester among women with PE/GH. The difference was statistically significant for second-trimester serum magnesium. The Wilcoxon signed-rank test showed a significant difference in mean serum magnesium values from the first to the second trimester in both the control group (P = 0.045) and the case group (P = 0.010).
| Parameters | Control Group | PE/GH Group | P-Value |
|---|---|---|---|
| Magnesium (mEq/L) | |||
| First trimester | 1.65 (1.24, 2.13) | 1.82 (1.59, 2.15) | 0.077 |
| Second trimester | 1.9 (1.48, 2.33) | 1.6 (1.25, 1.8) | 0.005 a |
| P-value | 0.045 b | 0.010 b | |
| NO (µmoles/L) | |||
| First trimester | 66.9 (53.06, 83.17) | 60.01 (32.9, 80) | 0.078 |
| Second trimester | 64.99 (47.7, 79.02) | 47.52 (35.11, 51.0) | 0.001 a |
| P-value | 0.373 | 0.141 |
Abbreviations: PE, pre-eclampsia; GH, gestational hypertension; NO, nitric oxide.
a P ≤ 0.05 is statistically significant between control group and PE/GH group.
b P ≤ 0.05 is statistically significant between first trimester and second trimester.
The Mann-Whitney test was also applied to assess the difference in median first- and second-trimester serum NO levels between cases and controls. Serum NO levels were lower in both trimesters among women with PE/GH, with the difference in the second trimester being statistically significant. However, the Wilcoxon signed-rank test indicated no significant change in median serum NO levels from the first to the second trimester within the control group (P = 0.373) or the case group (P = 0.141).
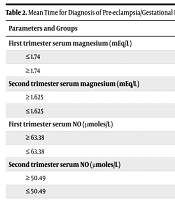
By ROC curve analysis, the cut-off values for disease prediction and the likelihood ratios for each parameter, along with sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV), were obtained. Table 2 presents the results of the ROC curve analysis. From the ROC curve analysis, it was observed that pregnant women with first trimester serum magnesium ≥ 1.74 mEq/L and second trimester serum magnesium ≤ 1.625 mEq/L are 1.3 and 1.8 times more likely, respectively, to develop the disease. Similarly, first trimester serum NO ≤ 63.38 µmoles/L and second trimester serum NO ≤ 50.49 µmoles/L were associated with 1.5 and 2.4 times greater likelihood, respectively, of developing the disease.
| Parameters and Groups | Median Time of Diagnosing Preeclampsia (wk) | 95% CI | P-Value a |
|---|---|---|---|
| First trimester serum magnesium (mEq/L) | 0.003 | ||
| ≤ 1.74 | 33.06 | 32.48, 33.64 | |
| ≥ 1.74 | 31.21 | 30.24, 32.19 | |
| Second trimester serum magnesium (mEq/L) | 0.000 | ||
| ≥ 1.625 | 32.96 | 32.39, 33.53 | |
| ≤ 1.625 | 30.98 | 29.85, 32.10 | |
| First trimester serum NO (µmoles/L) | 0.002 | ||
| ≥ 63.38 | 33.01 | 32.44, 33.6 | |
| ≤ 63.38 | 31.17 | 30.14, 32.21 | |
| Second trimester serum NO (µmoles/L) | |||
| ≥ 50.49 | 33.07 | 32.48, 33.65 | |
| ≤ 50.49 | 30.54 | 29.57, 31.51 | 0.003 |
Abbreviation: NO, nitric oxide.
a A P-value of ≤ 0.05 is considered statistically significant.
Cox regression analysis was used to determine the relative risk based on the cut-off values. Women with first trimester serum magnesium ≥ 1.74 mEq/L had a 2.8-fold increased risk, and those with second trimester serum magnesium ≤ 1.625 mEq/L had a 4.3-fold increased risk of developing the disease. These results were statistically significant. Furthermore, women with first trimester serum NO ≤ 63.38 µmoles/L had a 4.5-fold increased risk of developing the disease, which was also statistically significant. However, second trimester serum NO ≤ 50.49 µmoles/L was associated with a 1.5-fold increased risk, which was not statistically significant.
The combination of the above parameters was also evaluated as predictors of PE/GH, and the following combinations were found to be statistically significant: (A) first trimester magnesium ≥ 1.74 mEq/L and first trimester NO ≤ 63.38 µmoles/L showed a relative risk of 4.4 (95% CI: 1.91 - 10.12; P = 0.001); (B) Second trimester magnesium ≤ 1.625 mEq/L and second trimester NO ≤ 50.49 µmoles/L showed a relative risk of 3 (95% CI: 1.29 - 6.93; P = 0.011).
The present study also attempted to predict the time of onset of PE/GH using serum magnesium and NO levels from both trimesters. Statistically, both first and second trimester serum magnesium and NO levels were found to significantly predict the timing of disease onset (Table 2).
5. Discussion
In pregnancy, magnesium levels in the mother are found to decrease from the first to the third trimester. Alterations in calcium and magnesium levels are associated with the incidence of hypertensive disorders in pregnancy (7). Kanagal et al. suggest the measurement of serum magnesium as a diagnostic marker in the prediction of PE (8). Significantly lower serum magnesium levels have been reported in women with pregnancy-induced hypertension (PIH) (9, 10). Screening for magnesium deficiency could serve as a predictor, and treatment of hypomagnesemia may reduce the consequences associated with PE and GH (10). Any malfunction in magnesium metabolism severely affects endothelial function and induces endothelial damage, suggesting a correlation between magnesium levels and hypertension. Furthermore, increased blood pressure has been associated with hypomagnesemia (11). To the best of our knowledge, studies measuring first trimester magnesium levels and following up to assess the development of hypertensive disorders in pregnancy are limited. However, comparative data between PE/GH cases and controls are available.
The normal range for serum magnesium in the first trimester is 1.33 - 1.83 mEq/L, and in the second trimester is 1.25 - 1.83 mEq/L (12). In the present study, we observed that, compared to controls, serum magnesium levels among PE/GH cases were lower in the second trimester, while first trimester levels were higher. However, the values remained within the normal range. Therefore, statistical analysis was used to deduce cut-off values for both trimesters to differentiate the incidence of PE/GH.
Decreased calcium causes an increase in parathyroid hormone secretion, which elevates intracellular calcium. This induces smooth muscle contraction, leading to vasoconstriction and elevated blood pressure. Decreased magnesium contributes to hypocalcemia, further aggravating the process. However, calcium levels were not assessed in the present study to establish a direct association between calcium and magnesium in blood pressure regulation. Tavana and Hosseinmirzaei conducted a follow-up study similar to the present one with a case: Control ratio of 1:2 and a sample size of 500 (13). They also observed significantly lower magnesium levels in pre-eclamptic women well before the onset of disease. This suggests that decreased magnesium levels may exist from early pregnancy and could serve as a predictor for the development and pathogenesis of the disease.
Magnesium is known to regulate endothelial function by lowering vascular resistance, thereby helping to maintain blood pressure. Increased extracellular magnesium has been shown to reduce arteriolar tension through the activation of endogenous and exogenous vasodilators, leading to decreased blood pressure (14). In the present study, lower magnesium levels observed between 16 - 20 weeks of gestation are identified as a contributing factor to endothelial dysfunction and hypertension. Magnesium deficiency impairs endothelial growth and migration and increases inflammatory markers (4). Inadequate dietary intake of magnesium also negatively impacts endothelial health (11). Additionally, changes in extracellular magnesium can alter the production and release of NO (6). Thus, low magnesium levels affect NO production, alter angiogenesis, and induce endothelial dysfunction — cumulatively contributing to the pathogenesis of PIH.
Nitric oxide plays a crucial role in angiogenesis. Deficiency in endothelial NO synthase impairs angiogenic development, and studies have shown that increased expression of NO synthase or higher availability of NO promotes endothelial cell proliferation and maturation. Nitric oxide also supports the proliferation of fetal endothelial cells and the establishment of maternal-fetal vascular networks. Vascular endothelial growth factor (VEGF) requires NO for angiogenesis and induces NO synthesis via VEGF receptors on endothelial cells. Nitric oxide also regulates matrix metalloproteinases like MMP-9, which play a role in the invasion process of angiogenesis (15).
To the best of our knowledge, there are no established reference ranges for NO levels during pregnancy, and limited data are available on placental NO levels in placental dysfunction-related diseases like PE. Information on first trimester serum NO levels and subsequent development of hypertensive disorders is also sparse, although comparative data between PE/GH cases and controls exist. This study was thus undertaken to investigate serum NO levels in pregnancies complicated by PE and GH. Reduced NO levels are a potential marker of endothelial dysfunction. Our study found decreased NO levels in both trimesters among the case group, suggesting that endothelial dysfunction begins early in pregnancy, even if clinical symptoms emerge only after 20 weeks of gestation. Statistical analysis was used to deduce trimester-specific cut-off values to differentiate the incidence of PE/GH. Previous studies also report significantly lower plasma NO levels in PE compared to controls (16, 17). Since NO is a mediator of vasodilation, reduced levels may contribute to impaired placental hemodynamics associated with PE. Oxidative stress is another factor implicated in PE. Increased reactive oxygen species, in conjunction with reduced NO, may lead to vasodilatory dysfunction and subsequent hypertension. Decreased NO production coupled with oxidative stress contributes to vascular damage in PE (18).
5.1. Conclusions
Therefore, serum magnesium and NO levels may serve as early predictors for the detection of PE/GH. However, further validation of these findings is required before they can be implemented in clinical practice. The predictive differences and effectiveness of these biomarkers should be separately examined in PE and GH to better understand their utility in differentiating between the two hypertensive disorders of pregnancy.