1. Background
Both vitamin E (Vit E) and trimetazidine (TMZ) have an antioxidant capacity or action against oxidative stress mainly reactive oxygen species (ROS) on the microenvironments around the cell membrane (1). Many investigations have demonstrated that Vit E, with its antioxidant properties, guards against hepatotoxicity caused by carbon tetra chloride (CCl4) by promoting the activity of antioxidant enzymes and preventing lipid peroxidation (1, 2). According to several researchers, rats treated with Vit E alone showed normal liver histology. In contrast, rats exposed to oxidative conditions such as nitrosamine treatment showed changes in the liver's normal tissue, including cell necrosis and vacuolation. However, Vit E treatment of rats exposed to oxidative stress preserved the liver's framework and reduced hepatocyte necrosis (3).
Trimetazidine is used to treat angina pectoris caused by ischemia or oxidative stress on heart cells. In patients with coronary heart disease, a one-month treatment with TMZ significantly reduces the content of free radical oxidation products, lipid peroxides, and malondialdehyde in atherogenic low-density lipoproteins, according to reports. Trimetazidine also increases glutathione peroxidase (GPx) activity to counteract lipid peroxidation in plasma. Of course, TMZ does not directly eliminate free radicals, but it reduces the negative effects of their intense oxidation. When antioxidant enzymes are activated, this effect reduces the negative consequences of ischemia (4).
The anticancer agent doxorubicin (DOX) is noted for its selective therapeutic efficacy against various malignancies, nonetheless, its administration may cause detrimental injuries to the liver, blood vessels, kidneys, and other organs (5). It has been proven that DOX induces ROS (1). The liver, as the primary detoxifying organ with antioxidant activity, is the most vulnerable organ to DOX-induced injuries. The increase in ROS production caused by the continued administration of DOX has activated apoptosis and caspase 3, particularly in the liver. Therefore, in addition to necrosis of liver cells, DOX can also cause their apoptosis (6). The important molecular mechanism by which anticancer drugs induce apoptosis is the leakage of cytochrome c from mitochondria, which can lead to increased expression of apoptotic genes including the Bax gene (7).
It has been determined that measuring GPx, or any antioxidant enzymes, is not a reliable indicator of the body's oxidation or antioxidation status. Instead, measuring the total antioxidant capacity (TAC) can be a reliable indicator of the body's antioxidant reserve status. In our study, the decrease in TAC was interpreted as a negative sign because it indicates the presence of dangerous oxidation conditions in the body caused by DOX administration (8). Clinically, a variety of toxic anticancer treatment protocols with some common mechanisms are more or less harmful to the liver. Toxic agent injuries or liver failure can be severe enough to be fatal (3).
2. Objectives
The aim of this article was to find out whether TMZ-Vit E antioxidant cocktail compounds may protect mice against hepatotoxic anticancer administration.
3. Methods
3.1. Materials
The Vit E (Vit E from Health Aid, U.K) and TMZ (VASTAREL,trimétazidine dichlorhydrateand, France) and DOX (Cell Pharma, 50 mg per 25 mL) were prepared.
3.2. Animal Management
The present research project was conducted on 25 male BALB/c mice, each weighing approximately 20 g. For a period of 14 days preceding the study, all mice were caged and fed the same diet to reduce stress and ensure their health. Following a physical health evaluation, the mice were distributed at random into five experimental groups (n: 5) as Sham mice were administered orally with DMSO: PBS 1:1 solution in pH: 7.2 (1 mL/kg) intraperitoneal (i.p.) at the 4 and 11 days of the study. Doxorubicin: A cumulative dose of 25 mg/kg/intraperitoneal (i.p.) DOX on the days 12, 13 and 14 (9); Vit E: Two doses of 200 IU/kg Vit E on the days 4 and 11, PO (10) + a cumulative dose of 25 mg/kg/i.p DOX on the days 12, 13 and 14; TMZ: Ten mg/kg/day TMZ for 14 days, orally (11) + a cumulative dose of 25 mg/kg/i.p DOX on the days 12, 13 and 14; and Vit E-TMZ: Vitamin E (two doses of 200 IU/kg Vit E on the days 4 and 11, PO) + TMZ (10 mg/kg/day) + a cumulative dose of 25 mg/kg/i.p DOX on the days 12, 13 and 14. Our approach toward animals in terms of curing and treating them was in accordance with the regulation that was specifically designed to protect animals that were utilized for scientific purposes.
3.3. Biochemical Serum Evaluation
On day 15, the animals were euthanized using ketamine (100 mg/kg) and xylazine (10 mg/kg), with blood sampling. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT) were measured. These measures are indicators of liver (hepatotoxicity) lesions.
3.4. Oxidant and Antioxidant Status in the Liver Tissue
3.4.1. Total Antioxidant Capacity
About 100 mg of moist liver tissue was homogenized in 0.5 mL of ice-cold (10X assay buffer) and centrifuged at 10000 g for 15 minutes at 4°C. Then the supernatant was collected and transferred to fresh tubes. The tubes were placed on ice. Then the TAC protocol was executed.
3.4.2. Malondialdehyde Assay
The malondialdehyde assay (MDA) activity was evaluated by the appearance of a yellow color caused by the reaction of tetrazolium salt with superoxide anion in the liver tissue. The evaluation of these two indicators was done by the photometric method at 530 - 540 nm wavelength. The MDA also reacts with thiobarbituric acid to produce an adduct that can be measured fluorometrically.
3.5. RT-qPCR Evaluation for TNFα and Bax Gene Expression
3.5.1. Total RNA Isolation
RNA isolation kit (Megarena, Kiagenfaravar, Iran) extracted pure RNA from about 5 mg of liver. The samples were processed with the DNase of kit to inhibit DNA contamination. A UV-Vis spectrophotometer (IMPLEN, Germany) revealed the optical density at A260/A280 and A260/A230.
3.5.2. cDNA Synthesis
Reverse transcription reaction was performed using cDNA synthesis kit (Yektatajhiz, Iran). The cDNA Synthesis Kit (Yektatajhiz, Iran) was used for starting reverse transcription in the 20 μL reaction volume. All components were included 1 μg template RNA (11 μL), RevertAid RT 200 U/μL (1 μL), RiboLock RNase inhibitor 20 U/μL (1 μL), random hexamer primer (1 μL), dNTP mix 10 mM (2 μL) and reaction buffer 5X (4 μL). Then, samples were incubated for 10 minutes at 25°C, 60 minutes at 42°C and 5 minutes at 75°C (12).
After obtaining the sequence of Bax, TNFα, and beta-actin genes from the NCBI site, the specific primers of each gene were designed by the Primer Express program, and the sequence of the primers was blasted to confirm their accuracy and specificity.
Primer sequences for Bax gene were forward as 5′-CACCAGCTCTGA-GCAGATG-3′ and reverse as 5′-GCGAGGCGGTGA-GCACTCC-3′, and for TNFα gene were forward as 5′-GGCAGGTCTACTTTGGAGTCATTGC and reverse as 5′-ACATTCGAGGCTCCAGTGAATTCGG while the β-actin primers were as internal control. The forward for β-actin was 5′-GTGGGGCGCCCCAGGCACCA-3′ and the reverse was 5′-CTCCTTAATGTCACGCACGATTTC-3′. The specific bands for β-actin were 540 bp and for Bax was 516 bp under ultraviolet light.
3.6. Histopathology
In order to conduct histological and immunohistochemical examinations, the livers were transported in a 10% formalin buffer after each necropsy was performed. According to the results presented in Table 1, histopathological scoring of liver damage was carried out (13).
| Score | Leukocyte Infiltration in Lobule and Triad | Lobular Vacuolar Degeneration | Lobular Necrosis |
|---|---|---|---|
| 0 | No | No | No |
| 1 | Up to 5 cells | Randomly | Up to 5 cells |
| 2 | 5 - 15 cells | Up to 25% | 5 - 10 cells |
| 3 | 15 - 25 cells | 25 - 50% | 10 - 15 cells |
| 4 | 25 - 50 cells | 50 - 75% | 15 - 20 cells |
| 5 | Over than 50 cells | Over than 75% | Over than 20 cells |
3.7. Statistical Analyses
The findings from the study were illustrated using GraphPad Prism9. software as mean ± SEM and analyzed using one-way analysis of variance (ANOVA), followed by Bonferroni's test for parametric data and Kruskal-Wallis one-way ANOVA for non-parametric data. The P-value less than 0.05 was considered to be statistically significant.
4. Results
4.1. Clinical and Gross Appearance
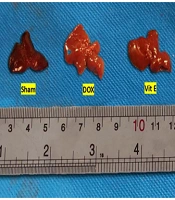
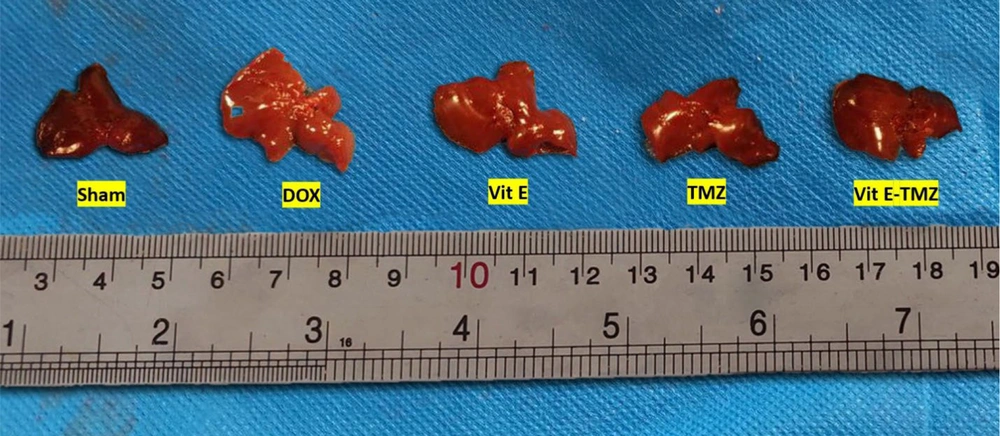
Mice given Vit E or TMZ maintained a healthy weight and overall condition throughout the duration of the experiment. In contrast, mice treated with DOX experienced numerous side effects, including matted hair, poor body condition, loss of activity, and weakness. After the rats were euthanized in the CO2 gas chamber, the gross appearances of the livers were compared. The DOX group had larger and paler livers than the sham and both Vit E-TMZ groups. The Vit E and TMZ groups also showed an intermediate state of liver injuries (Figure 1).
Gross appearance of the liver of BALB/c mice related to doxorubicin (DOX)-induced hepatopathy, which was protected via vitamin E (Vit E) and trimetazidine (TMZ) treatments. The livers administered DOX were paler and larger than sham. The co-administration of Vit E and TMZ helped to reduce those appearances.
4.2. Biochemical Parameters
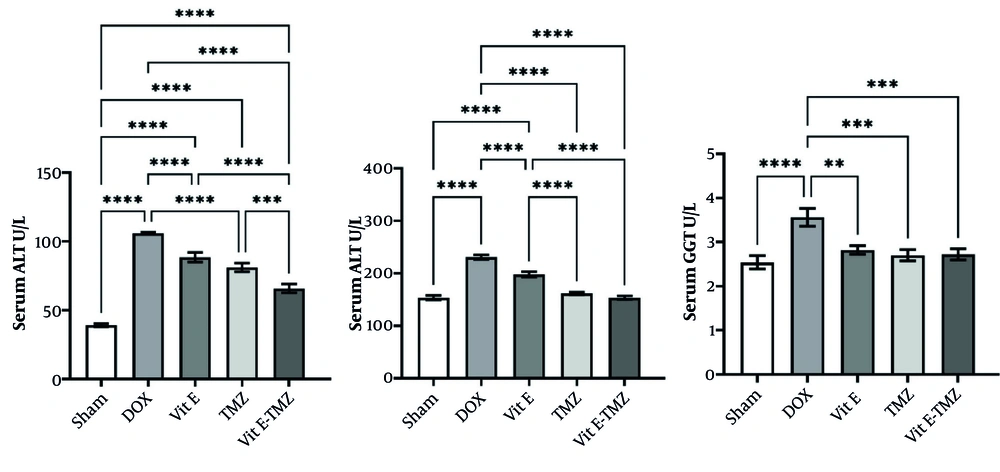
The ALT, AST, and GGT activities in the DOX group were significantly higher than those in other groups. In ALT, a very significant difference was seen between the sham groups and other treatments with the DOX group (P < 0.0001). There was no significant difference between the two groups of Vit E and TMZ treatments (P > 0.05). On the other hand, there was a very significant difference between these two groups and the Vit E-TMZ group. These results showed that first DOX with a cumulative dose of 25 mg/kg/bw has very destructive effects on liver tissue. Second, the administration of antioxidants such as Vit E and TMZ can reduce the severity of DOX-related injuries. It was also found that the combined administration of both Vit E and TMZ, the group of Vit E-TMZ, can prevent the increase of ALT level induced by DOX (Figure 2).
Effects of vitamin E (Vit E) and trimetazidine (TMZ) on liver enzymes. The co-administration of Vit E and TMZ resulted in a reduction of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT) enzyme levels (** P < 0.01, *** P < 0.001, **** P < 0.0001).
In AST, it was observed that the sham group has a significant difference (P < 0.0001) with the DOX group. On the other hand, the treatment groups also had a significant difference with the DOX group. Importantly, there was no significant difference between TMZ and both Vit E-TMZ groups with the sham group (P > 0.05). On the other hand, there was a significant difference between the Vit E and TMZ groups. These results showed that DOX put a lot of pressure on the liver to increase the AST enzyme. Also, antioxidants, including Vit E and TMZ, can help reduce the amount of AST depending on the severity of liver injuries. However, the two groups of TMZ and both Vit E-TMZ were able to reduce the severity of AST much more than Vit E, and this indicates a greater and stronger effect of TMZ reducing liver injuries.
In GGT, it was observed that there is a significant difference between the sham and DOX groups. Also, the treatment groups including Vit E, TMZ, and both Vit E-TMZ also had a significant difference (P < 0.0001) with the DOX group. The point here was that no significant difference was observed between the different treatment groups, and on the other hand, all of them did not have a significant difference with the sham group (P > 0.05). This means that Vit E, TMZ, and Vit E-TMZ can effectively help the liver so that the serum level of GGT does not rise due to its injuries (Figure 2).
4.3. Total Antioxidant capacity and Malondialdehyde Assay Evaluation
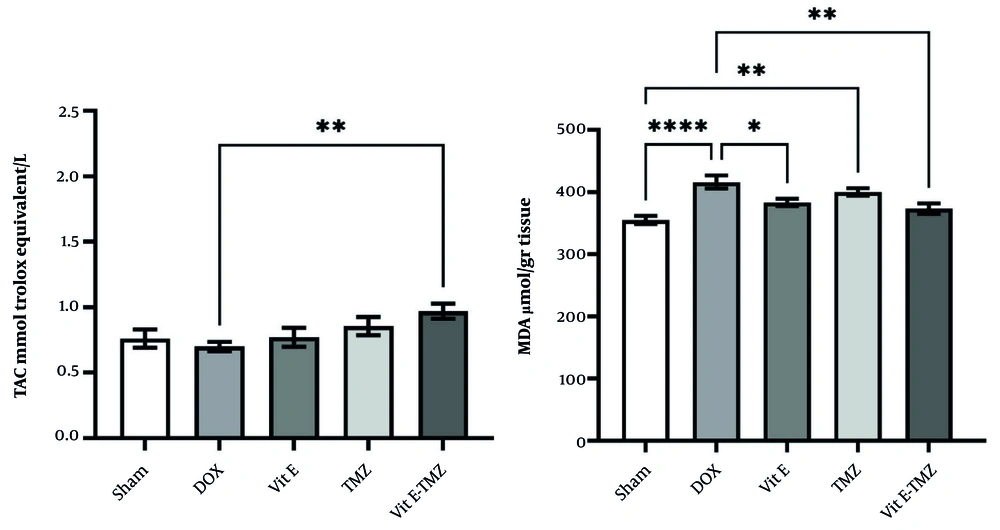
The TAC in the DOX group compared to the sham and other groups except the Vit E-TMZ group (P < 0.01) did not show a significant difference (Figure 3). These results mean that the dose of 25 mg/kg/bw of DOX has no effect on reducing the serum TAC level in a period of 48 hours. On the other hand, the group of both Vit E-TMZ showed a significant difference with the DOX group, which indicated the increase of TAC in the liver of these mice more than the other groups (Figure 3).
Effects of vitamin E (Vit E) and trimetazidine (TMZ) on the antioxidants in doxorubicin (DOX)-induced liver lesions. There are significant differences among the treating compared with the sham group in the total antioxidant capacity (TAC) and malondialdehyde assay (MDA) assays (* P < 0.05, ** P < 0.01, **** P < 0.0001).
In the MDA test, there was a significant difference between the sham group and DOX (P < 0.0001). On the other hand, the groups receiving Vit E had a moderately significant difference with the DOX group (P < 0.01). However, no significant difference with DOX (P > 0.05) was observed in TMZ group. Also, there was a significant difference between the group receiving TMZ and the sham group (P < 0.01). These results show that the effect of Vit E in eliminating cellular oxidation in the liver was greater than that of TMZ. Therefore, Vit E was more effective than tramazoline in controlling DOX-induced cytotoxicity (Figure 3).
4.4. Gene Expression
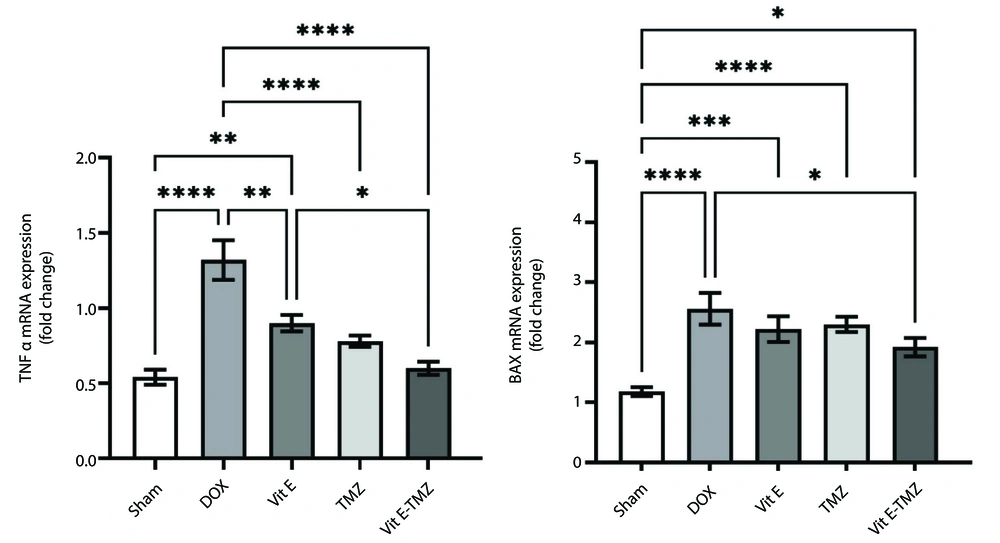
The results obtained from qRT-PCR analysis are shown in Figure 4. Doxorubicin can increase the expression of TNFα and Bax genes (P < 0.0001).
Regarding TNF-alpha, a significant difference was observed between the control and DOX treatment groups. However, the significance level between the Vit E group and the DOX was lower than the others. Although there was no significant difference between the groups receiving TMZ and both, there was a significant difference between the treatment with Vita E and both. Based on these findings, we concluded that DOX greatly increases TNF-alpha levels and inflammation in the liver. In Contrast, the medicine TMZ appears to be more effective in reducing inflammation than Vit E. However, Vit E and TMZ together gave the best results.
In the Bax gene evaluation, although the biggest difference was seen between the sham group and the other groups (P < 0.0001), among the treatment groups, the Vit E-TMZ group had the least significant difference from the sham group (P < 0.05). The three groups of DOX, Vit E, and TMZ were not significantly different. These results indicated the high apoptotic effect of the DOX drug. On the other hand, treatment groups other than the simultaneous use of Vit E and TMZ could not prevent apoptosis optimally. Even though the Vit E-TMZ group reduced the amount of apoptosis to the desired level, it was not seen either in the Vit E or TMZ administration alone (Figure 4).
4.5. Histopathology
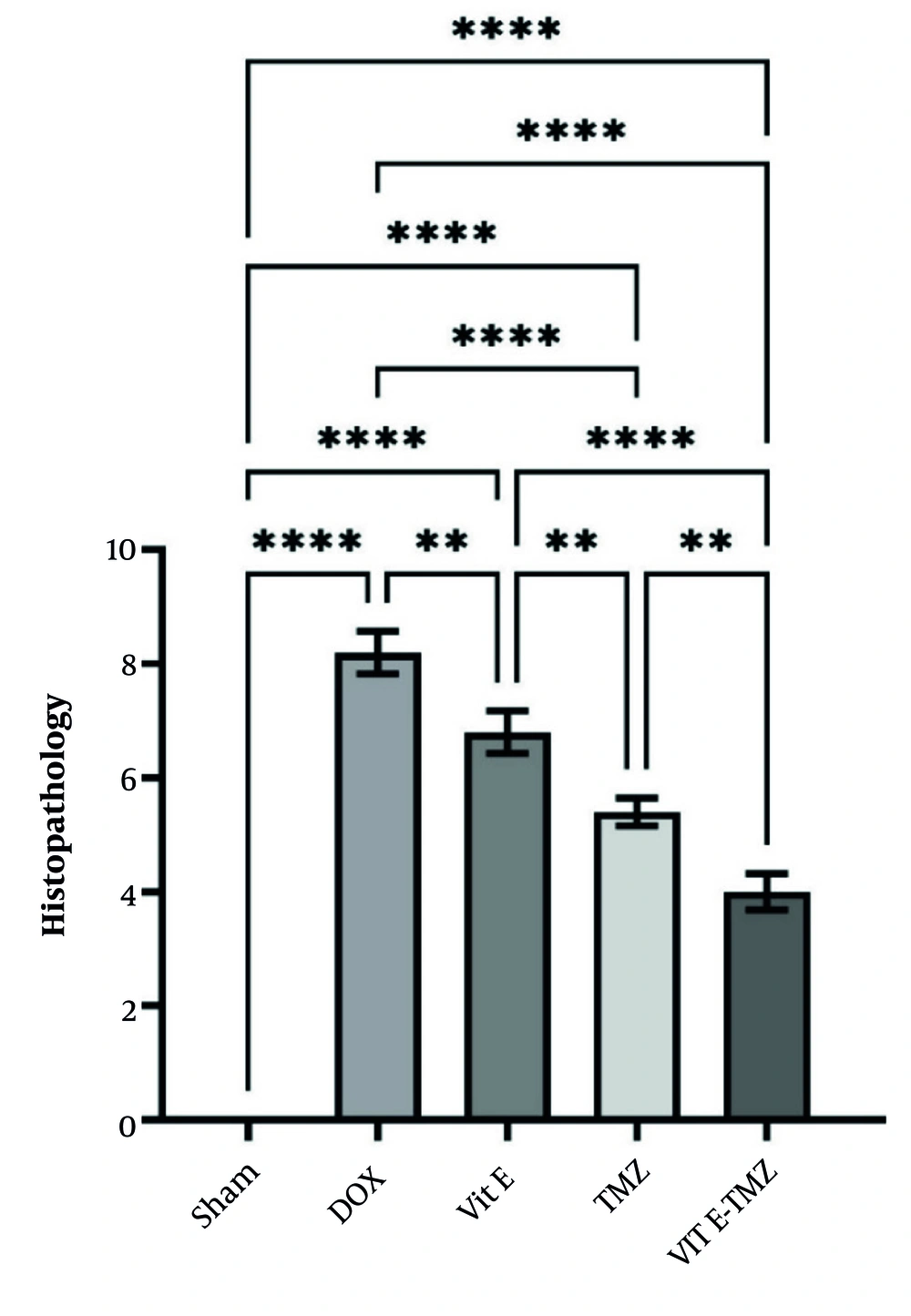
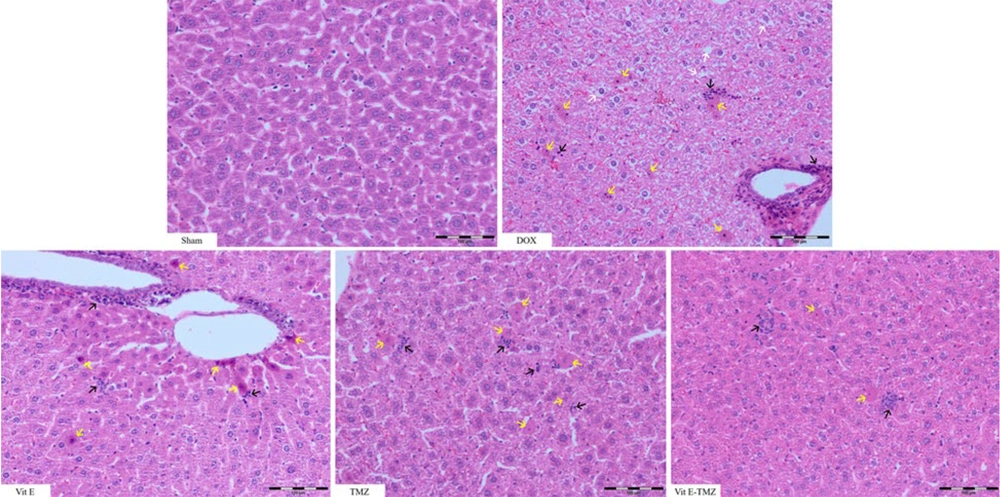
In the sham group of mice, the liver section had normal lobules made up of straight columns of hepatocytes that go from the center vein to the edges of the portal areas. Significant differences were observed among all groups regarding necrosis, inflammation, and degeneration, particularly between the sham and DOX groups (P < 0.0001). All study groups demonstrated significantly superior outcomes compared to the DOX group (P < 0.01).
In the treatment groups receiving TMZ compared to Vit E, more improvement of liver injuries was observed due to the fact that it had a lower histopathological score (P < 0.01). The simultaneous administration of Vit E and TMZ significantly improved the liver tissue more than the administration of each alone (Figures 5 and 6).
5. Discussion
Doxorubicin is a strong chemical that generates a significant amount of free radicals in liver cells. Antioxidants stored in the body are unable to eliminate them through the liver (11). Doxorubicin metabolism by hepatic enzymes abundant in mitochondria leads to hepatocyte destruction (14). Increasing ROS, lowering mitochondrial oxidation cycles, chromatin malformation and breakdown, and necrosis or apoptosis are all signs of liver damage caused by DOX (15).
Recently, Cella et al. investigated the effects of DOX administration on body weight, muscle volume, and physical mobility and announced that animals receiving DOX experienced cachexia, muscle loss or shrinkage, and became very weak. To evaluate sarcopenia, Cella et al. measured the stretch and histological atrophy of the gastrocnemius muscle (16). In the present study, all mice treated with DOX suffered from poor body condition, loss of activity, and weakness. The DOX group had larger and paler livers than the sham group. Vitamin E and TMZ groups showed a moderate level of liver injuries.
In a study assessing biochemical parameters and conducting histopathological evaluations in rats treated with cisplatin, Ateyya et al. concluded that TMZ offers hepatoprotective effects (17).
Their results showed that the serum levels of liver enzymes, including AST, ALT, and gamma glutamyl transferase (γ-GT), were increased and microscopic changes were also seen in the liver structure. There are also significant changes in the antioxidant defense system, which was shown by a significant decrease in total antioxidants and reduced liver levels of glutathione (GSH) and superoxide dismutase (SOD), along with a significant increase in lipid peroxidation. Significant increases in hepatic nuclear factor kappa B (NF-κB) activity and hepatic Bax protein expression were observed. Finally, they concluded that TMZ provides protection against liver injuries caused by cisplatin by removing oxygen-free radicals and having anti-inflammatory and anti-apoptotic effects, as well as reducing NF-kB activation (17).
In the present research, ALT, AST, and GGT activities were significantly higher in the DOX group than in other groups. For the ALT, the significant difference was observed between the sham and treatment groups with the DOX group. There was a highly significant difference between Vit E or TMZ groups and the Vit E-TMZ group. These results showed that the combined administration of Vit E and TMZ, Vit E-TMZ group, could prevent the increase in DOX-induced ALT level, which is characteristic for better-improved liver.
All treatment groups showed a significant difference in AST levels compared to the DOX group.
Considering the lack of significant difference between TMZ and both Vit E-TMZ groups, alongside a significant difference form the Vit E group, we concluded that TMZ has greater and more effective impact compared to Vit E in reducing AST level and liver injuries.
The findings of our research about MDA evaluation revealed that the effect of Vit E in eliminating cellular oxidation in the liver was greater than that of TMZ. Therefore, Vit E was more effective than TMZ in controlling DOX-induced cytotoxicity.
In 2022, in a study on the protective effects of TMZ with antioxidant and anti-inflammatory properties on the bladder of mice treated with cyclophosphamide, Engin et al. concluded that the amount of MDI of inflamed urinary bladder tissue in the group receiving TMZ was significantly lower than the group receiving cyclophosphamide (18).
The findings of our research about MDA evaluation revealed that the effect of Vit E in eliminating cellular oxidation in the liver was greater than that of TMZ. Therefore, Vit E was more effective than tramazoline in controlling DOX-induced cytotoxicity.
In a study conducted by Chen et al. in 2016 on pancreatitis, TMZ administration significantly increased serum TAC levels compared to the untreated pancreatitis group, indicating that TMZ can significantly reduce oxidative stress (19). Similar results were evaluated by Mahfoudh-Boussaid et al. in 2012 regarding the significant reduction of oxidative stress and improvement of renal function in Wistar rats by TMZ (20). In 2023, Zhang et al. demonstrated that the spontaneous remodeling of the atria induced by high-fat stimulation plays a key role in the development of atrial fibrillation. rimetazidine prevented neuronal remodeling by inhibiting oxidative stress to reduce the occurrence of atrial fibrillation by increasing eNOS expression (21).
In 2016, Ma et al., reported that TMZ provides protection against cardiac ischemia-reperfusion (I/R) injury in mice by modulating the cardiac Bcl-2/Bax pathway (22). In 2010, Ciftel et al. studied the protective effects of TMZ and dexmedetomidine against liver injury induced by mesenteric artery I/R through endoplasmic reticulum stress (ERS) mechanisms and reported that the increased levels of AST, ALT, creatinine, and the increased expression of caspase 3 and Bax were significantly reduced by the administration of TMZ and dexmedetomidine. Also, pathological I/R injuries, including necrosis, inflammation, and significant liver congestion, were reduced after the administration of these two drugs. These effects are likely mediated through reduction of ERS and apoptosis. It is noteworthy that dexmedetomidine showed fewer protective effects than TMZ (23).
Evaluation of Bax gene expression in our study revealed that, except for the group receiving combined Vit E and TMZ, the other treatment groups were not effective in preventing apoptosis.
The Vit E-TMZ group was able to reduce the rate of apoptosis, which was not seen in the use of Vit E or TMZ alone. The present study showed that while both Vit E and TMZ treatments could effectively reduce TNF-α gene expression, the reduction level of TNF-α in the TMZ-Vit E group was significantly lower than either alone. This indicated that the combination of Vit E and TMZ could more effectively reduce inflammatory stress in DOX toxicity.
Abdelgawad et al. investigated the effects of 4-week administration of DOX (4 mg/kg/week) on serum, heart, liver, and kidneys of C57BL/6N mice with lymphoma tumors (EL4 mice). They showed that treatment with DOX suppresses tumor growth in tumor-bearing mice but also causes significant cardiac atrophy. Interestingly, DOX significantly improved the severe inflammatory response induced by the tumor in the heart, liver, and kidney. The results of Abdelgawad's work differed with the present study in terms of the intensity of inflammation, which is probably related to the fact that the mice were not tumor carriers (24).
By investigating the preventive effects of TMZ on the chronic pancreatitis mouse model, Kaplan et al. showed that the biochemical parameters of the groups receiving TMZ were significantly more favorable compared to the chronic pancreatitis group. Additionally, although the difference between the low-dose TMZ group (5 mg/kg) and the chronic pancreatitis group was not significant in terms of histopathological scores, the difference between the high-dose TMZ group (10 mg/kg) and the chronic pancreatitis group was significant. These Kaplan results showed that TMZ has the ability to reduce liver lesions such as necrosis, vacuolation, and inflammation (25).
In this study, there was a significant difference between all groups in necrosis, inflammation, and degeneration, especially in the sham versus DOX group. In the treatment groups, it was observed that TMZ reduced inflammation and necrosis in the liver compared to Vit E. The simultaneous administration of Vit E and TMZ significantly improved the liver tissue more than the administration of each alone.
5. Conclusion
Trimetazidine and Vit E, when administered individually, exhibit antioxidant and hepatoprotective properties. However, their combined use offers enhanced protective effects, including greater reductions in liver inflammation, degeneration, necrosis, and apoptosis.