1. Introduction
Contrast-induced neurotoxicity (CIN) is a medical complication that manifests as clinical symptoms of ischemic stroke after the administration of iodinated contrast, and due to the increase in the use of angiographic studies in endovascular interventions, its incidence is increasing (1-3). Iodinated contrast agents have been employed in medical imaging since the 1920s, initially introduced in carotid angiography (4). Over time, the development of low-osmolality and low-viscosity non-ionic contrast agents has substantially reduced their toxicity (4, 5). Common adverse reactions include idiosyncratic effects such as nausea, vomiting, generalized weakness, and anaphylaxis, as well as dose-related, organ-specific complications like contrast-induced nephropathy (5). Neurological side effects, although rare, have been increasingly reported, particularly in the context of cardiovascular imaging, with CIN being recognized more frequently (5-7). In this report, we present an unusual case of unilateral neurological complications following contrast administration and briefly review the literature on current management strategies.
2. Case Presentation
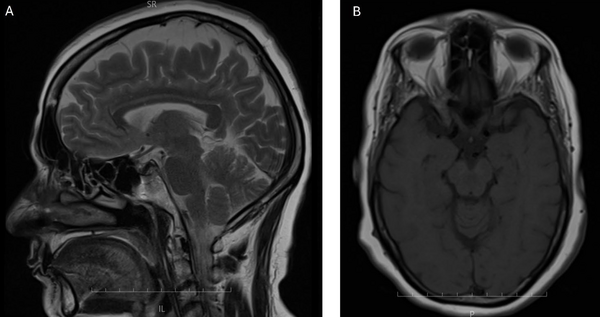
A 51-year-old female was admitted to our tertiary care center with a two-month history of exertional chest pain and dyspnea (NYHA FC I-II). Her past medical history was significant for hypertension and a familial predisposition to coronary artery disease, both managed with medications including ASA 80 mg, rosuvastatin 10 mg, amlodipine/valsartan 5/80 mg, eplerenone 25 mg, and bisoprolol 2.5 mg twice daily. Additionally, she was being treated for rheumatoid arthritis with prednisolone 5 mg twice daily, hydroxychloroquine 200 mg, and methotrexate 15 mg weekly. Based on her clinical presentation, medical history, and reduced ejection fraction on echocardiography, she was scheduled for coronary angiography. The angiography, performed via the right radial artery using 30 cc of IOPAQUE (IOPAQUE 300MG I/ML 20ML AMP), revealed patent coronary arteries, and the patient was advised to follow up with medical management. However, 30 minutes post-procedure, she developed incontinence and flaccid quadriplegia while remaining fully conscious and oriented. On neurological examination, the patient was fully conscious and oriented to time, place, and person. Cranial nerves II–XII were intact; pupils were equal and reactive. Motor testing revealed grade 0/5 strength bilaterally in both upper and lower extremities, with flaccidity and no voluntary movement. Light touch and pinprick sensations were globally diminished — assessment limited by motor deficit. Deep tendon reflexes were initially hypoactive in all limbs. Over the next six hours, motor strength in the upper extremities improved to grade 3/5 and to grade 4/5 in the lower extremities; by 48 hours post-onset, strength returned to grade 5/5 and reflexes normalized. An immediate MRI ruled out any infarction or acute lesions (Figure 1), and subsequent angiography showed no carotid or cerebral obstruction (Figure 2). These findings were suggestive of CIN. The patient was administered 200 mg of hydrocortisone both before and after the procedure, and conservative management with intravenous saline hydration was initiated. Her symptoms gradually resolved within six hours, with full recovery occurring over 48 hours. The patient was discharged in good health and remained symptom-free at follow-up visits one month, six months, and one year post-discharge.
3. Discussion
Although neurological complications after iodinated contrast administration are overall uncommon, they have been reported more frequently in the setting of cardiovascular angiography procedures (5, 7). Knowing the clinical symptoms of CIN plays an important role in its diagnosis. Previous studies have reported symptoms including encephalopathy, cortical blindness, motor and sensory deficits, aphasia, seizures, headaches, and death for CIN (7-10), (11-14). While the underlying mechanism remains unclear, it is thought that disruption of the blood-brain barrier (BBB) by hyperosmolar contrast agents may play a role (7, 15). This disruption may result from osmotic shrinkage of neurons and separation of endothelial tight junctions, leading to increased intraluminal tension and vasodilation (5, 6, 15). Additionally, contrast injury may affect regions of the CNS lacking a BBB, such as the hypothalamus (16). Symptoms such as nausea and vomiting may result from effects on the medullary area postrema, while vasovagal responses and hypotension may be attributed to carotid receptor involvement (5). Hyperosmolar reactions, leading to cellular fluid efflux, can manifest as malaise and generalized weakness, and seizures may occur due to irritation of unprotected brain regions by the contrast agent. Other manifestations, such as paresthesia and myoclonus, may arise as delayed spinal cord responses, and transient cortical blindness could result from direct neurotoxicity affecting the occipital cortex (5). Cortical blindness has been reported as a prominent manifestation of CIN in some studies, with one review of 33 cases following coronary angiography reporting an incidence as high as 58%. However, it is important to note that this is based on a limited number of reviewed cases, and earlier studies of vertebral angiography reported a much lower incidence, ranging from 0.3% to 1% (17). Patients with diabetes, hypertension, and hyperlipidemia are particularly susceptible. It is hypothesized that cortical blindness may be underreported due to its transient nature and lack of patient awareness (5). While most brain CT scans in these cases appear normal, abnormal bilateral occipital contrast enhancement has been observed (5). Isopaque, an iodinated contrast agent, has been noted in some literature to be associated with cortical blindness, though a definitive dosage correlation remains unclear. In our case, cortical blindness was not observed. Mental status changes are reported as the second most common neurological adverse effect of CIN after coronary angiography, occurring in about 24% of cases (5). Although higher contrast doses have been suggested to increase neurotoxic risk, the relationship between dose and mental status alterations remains unproven; in our case, cognitive function was preserved. Additional CIN-related outcomes documented in the literature are seizures (5%), spinal myoclonus (1%), paralysis (7%), and coma (1%) (5). Although two cases of paralysis following iodixanol use have been reported (18), our patient presented with hemiparesis and ipsilateral paresthesia about 30 minutes after receiving 30 mL of IOPAQUE. Because no consensus therapeutic protocol for CIN exists, treatment is principally supportive, focusing on hydration and close neurological monitoring (5, 6). Management typically focuses on supportive care, including monitoring blood pressure, fever, and electrolytes (6). Anticonvulsants have shown potential efficacy in treating seizures and myoclonus associated with contrast administration (5). Experimental animal studies suggest that pre-treatment with low molecular weight dextran and corticosteroids may reduce red blood cell aggregation, decrease BBB permeability, and prevent CIN (19, 20). Our patient’s neurological deficits fully resolved over 48 hours under conservative treatment — 0.9% saline hydration plus 200 mg hydrocortisone. While isolated cases cite cumulative doses above 100 mL as a risk factor for CIN (5), the overall evidence remains inconclusive. Indeed, paralysis has occurred after administration of only 30 mL of contrast.
3.1. Conclusions
Contrast-induced neurotoxicity, while uncommon, is a clinically important complication of angiography that necessitates heightened awareness. Given the variable manifestations of CIN, which can include cortical blindness, clinicians should consider this diagnosis even in the absence of typical risk factors or with lower contrast volumes. Our case underscores the unpredictable nature of CIN, with paralysis occurring after a minimal 30 cc Isopaque dose, yet resolving fully with supportive care. Future research should prioritize elucidating the mechanisms of CIN to guide preventative and treatment approaches.