1. Background
Aortic stenosis (AS) is a gradual disease characterized by a prolonged asymptomatic phase, often leading to a swift deterioration once symptoms emerge (1). This results in a significant mortality rate, with untreated individuals facing approximately a 50% chance of death within the first two years after symptom onset (2-4). Surgical aortic valve replacement (AVR) is the standard treatment for this condition, providing symptom relief and improved survival rates in affected patients (5). Moreover, when there are no severe accompanying health issues, the procedure typically has a low risk of operative mortality (6-8). Nevertheless, clinical observations indicate that at least 30% of patients with symptomatic, severe AS do not receive valve replacement surgery. This can be attributed to factors such as advanced age, impaired left ventricular function, or the presence of several comorbid conditions (9-11).
For these high-risk patients, exploring less invasive treatment options may be beneficial (12). Based on established research, transcatheter aortic valve implantation (TAVI) has emerged as the standard approach for treating patients with symptomatic severe AS who also have serious comorbidities that disqualify them from undergoing open surgical AVR (2, 13). The inaugural TAVI procedure was conducted by the pioneering physician Alain Cribier in 2002, and the technique has since been developed and implemented on a large scale, with thousands of successful cases worldwide (14).
Although adequate patient selection is a crucial component of TAVI, it remains a complex clinical challenge. Ensuring that the transcatheter procedure is performed on the right patients — those likely to gain functional and survival benefits — is vital to avoid subjecting individuals to unnecessary high-risk interventions and their associated costs (1, 15-17). However, determining patient eligibility can be intricate due to the significant comorbidities frequently present in TAVI candidates, which complicates the prediction of beneficial treatment outcomes for specific individuals. This complexity is further exacerbated by the reduced reliability of surgical risk assessment tools such as the EuroSCORE and Society of Thoracic Surgery score in the TAVI population (18-20). Moreover, there is still a gap in the comprehensive understanding of the predictors that could influence procedural mortality and morbidity. In November 2015, the Balloon-Expandable Valve method for TAVI was standardized at the Shahid Modarres Educational and Therapeutic Center.
2. Objectives
The purpose of this study was to assess the rates of 30-day and 6-month mortality, as well as procedural morbidity, following the TAVI procedure.
3. Methods
3.1. Study Participants
This retrospective cross-sectional study was conducted at Shahid Modarres Educational and Therapeutic Center, an affiliated hospital of Shahid Beheshti University of Medical Sciences. The study population consisted of a total of 100 consecutive cases with severe, symptomatic AS who were selected by the Heart Team in the recent 3 years (2021 - 2024) and were included in the present investigation. Notably, the procedure was modified in the last three years, with patients undergoing TAVI under conscious sedation via transfemoral access with fluoroscopic guidance (21).
The inclusion criteria were patients with severe, symptomatic AS confirmed by echocardiography [mean pressure gradient (MPG) ≥ 40 mmHg or aortic valve area (AVA) ≤ 1.0 cm2] who were deemed high-risk for surgical AVR due to comorbidities, advanced age, or impaired left ventricular function, as assessed by the Heart Team. Exclusion criteria included patients with active endocarditis, severe untreated coronary artery disease requiring immediate intervention [non-significant coronary artery disease (CAD) where the TAVI procedure is performed], or life expectancy < 1 year due to non-cardiac conditions. The Heart Team, comprising interventional cardiologists, cardiac surgeons, and imaging specialists, made the final decision on TAVI eligibility based on clinical, echocardiographic, and computed tomography (CT) angiography findings.
All participants underwent comprehensive pre-procedural evaluations, including transesophageal echocardiography (TEE) and CT angiography, to assess aortic valve morphology and annulus sizing. Following the TAVI procedure, TEE was repeated to evaluate valve function and detect complications. The TEE was utilized pre- and post-procedure to enhance procedural accuracy and safety, providing real-time guidance for valve deployment and immediate identification of complications such as paravalvular leakage (PVL), MPG, peak pressure gradient (PPG), or valve malpositioning. This approach was particularly critical during the early learning curve of our TAVI program, initiated in 2015, with a planned transition toward greater reliance on transthoracic echocardiography (TTE) as our experience grows. This study was conducted in accordance with the accepted regulations of the Shahid Beheshti University of Medical Sciences Ethical Committee under the code (IR.SBMU.RETECH.REC.1403.806). Informed consent was obtained from all participants.
3.2. Transcatheter Aortic Valve Implantation Procedure
Prior to the procedure, a comprehensive evaluation was conducted using TEE to assess key cardiac factors such as left ventricular ejection fraction (LVEF), left ventricular (LV) hypertrophy, and the type of aortic valve, distinguishing between tricuspid and bicuspid varieties. The severity of AS was analyzed through measurements of pressure gradients (PG and MG), in addition to calculating the AVA and measuring the aortic annulus size. The evaluation also included assessments of aortic insufficiency and the functionality of other heart valves, as well as pulmonary artery pressure (PAP) and additional important cardiac attributes. On the other hand, CT angiography focused on identifying potential coronary artery issues, determining the distance of the left main coronary origin from the aortic annulus, and examining the thoracic aorta along with the shape and diameter of the aortic arch.
The TAVI was performed using a transfemoral approach for all patients. In the contralateral leg, a 6 Fr venous and arterial sheath was placed to facilitate the implantation of a temporary pacemaker lead at the right ventricular (RV) apex, along with an arterial pigtail catheter for aortography during the delivery of the prosthetic valve. Following a successful femoral arteriotomy and the insertion of a specialized 22 Fr sheath, rapid pacing of the right ventricle was tested until arterial pressure dropped below 50 mmHg with a heart rate of 180 to 200 bpm. Aortography was conducted using the pigtail catheter to identify an optimal position for prosthetic aortic valve delivery. A favorable position was indicated by the alignment of the three aortic cusps and an adequate distance between the left main coronary artery and the annulus.
After careful verification, balloon aortic valvuloplasty was performed under rapid pacing, accompanied by simultaneous aortography. This step aimed to facilitate the opening of the aortic valve while observing left main flow, the presence of aortic insufficiency, and ensuring proper sizing of the aortic annulus. These parameters were monitored using TEE. Subsequently, a balloon-expandable bioprosthetic aortic valve was prepared using a specialized delivery system, with the balloon inserted through the previously placed 22 Fr sheath over a 0.035 super stiff guide wire in the left ventricle. The valve was advanced from the aortic arch and through the native aortic valve with caution.
Positioning was then re-evaluated through additional aortography and TEE. Finally, under rapid RV pacing at a rate of 180 to 200 bpm and with real-time aortography, the prosthetic valve was implanted in the aortic annulus using rapid inflation of the inflator. Post-procedure, final aortography was conducted to assess the function of the prosthetic valve, checking for aortic insufficiency and left main flow, as well as potential complications such as aortic dissection. The TEE was also employed to evaluate the position and function of the prosthetic valve, with particular attention given to the valve gradient.
3.3. Statistical Analyses
Quantitative factors were presented as mean ± standard deviation (SD), while qualitative factors were presented as numbers with corresponding percentages. To compare qualitative variables between groups, the chi-square test was performed, and the t-test was applied to compare quantitative factors. Additionally, the correlations between indexes were calculated using Pearson's r coefficient for normally distributed variables and Spearman's rho for non-normally distributed variables. All statistical analyses were conducted using SPSS 24 software, and the corresponding graphs were generated using GraphPad Prism 8.4.2 (GraphPad Software Inc.). For this study, a P < 0.05 was considered statistically significant.
4. Results
4.1. Characteristics of Patients
Table 1 presents the demographic and clinical characteristics of the patient cohort. The average age of the participants was 77.82 ± 6.97 years, consisting of 55% male participants. The chief complaints noted were dyspnea on exertion (DOE) in 73 patients, chest pain (CP) in 8, syncope in 1, and a combination of DOE and CP in 18 patients. The most common CAD classification was single-vessel disease (SVD) (12 cases). Society of Thoracic Surgeons (STS) score, which indicates the surgical risk, averaged 7.22 ± 2.19, reflecting a moderate risk profile within this group.
| Parameters b | Values |
|---|---|
| Gender (male) | 55 |
| Hypertension (yes) | 75 |
| Diabetes (yes) | 41 |
| Hyperlipidemia (yes) | 51 |
| Smoking (yes) | 15 |
| ECG (sinus) | 84 |
| PCI (yes) | 32 |
| CABG (yes) | 13 |
| BMI (kg/m2) | 26.7 ± 4.2 |
| Age (year) | 77.82 ± 6.97 |
| STS score | 7.22 ± 2.19 |
| CAD | |
| SVD | 12 |
| 2VD | 3 |
| 3VD | 3 |
| Mild CAD | 6 |
| Chief complaint | |
| DOE | 73 |
| CP | 8 |
| Syncope | 1 |
| DOE + CP | 18 |
| Weight (Kg) | 71.87 ± 12.95 |
| Height (cm) | 164.11 ± 8.38 |
Abbreviations: ECG, electrocardiogram; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; STS score, society of thoracic surgeons score; CAD, coronary heart disease; SVD, single vessel disease; 2VD, double vessels disease; 3VD, triple vessels disease; DOE, dyspnea on exertion; CP, chest pain.
a Values are expressed as mean ± SD.
b Because the number of patients in the study is 100 cases. The number of qualitative variables is equal to the percentage and is not mentioned.
Table 2 summarizes the echocardiographic findings of patients before the procedure. A significant majority of patients exhibited normal left ventricular (LV) size (82%), while left ventricular enlargement (LVE) was noted in a smaller cohort with mild LVE (12%), and very few cases of moderate (5%) to severe (1%) LVE. The RV size was predominantly normal (90%), although mild (9%) to moderate (1%) RV enlargement (RVE) was observed. The RV function was primarily normal (84%), and mild RV dysfunction was present in 11% of patients. Most patients had normal mitral valves (84%), with a small percentage exhibiting varying degrees of mitral stenosis and regurgitation. Furthermore, notable measurements included an average AVA of 0.72 ± 0.17 cm2, an ejection fraction (EF) of 51.7 ± 9.04%, and a systolic pulmonary artery pressure of 39.49 ± 11.59 mmHg.
| Parameters | Values |
|---|---|
| LV size | |
| Normal | 82 |
| Mild LVE | 12 |
| Moderate LVE | 5 |
| Severe LVE | 1 |
| RV size | |
| Normal | 90 |
| Mild RVE | 9 |
| Moderate RVE | 1 |
| RV function | |
| Normal | 84 |
| Mild RV dysfunction | 11 |
| Moderate RV dysfunction | 5 |
| Mitral stenosis | |
| Normal | 84 |
| Mild | 10 |
| Moderate | 1 |
| Severe | 5 |
| Mitral regurgitation | |
| Normal | 5 |
| Mild | 35 |
| Mild to moderate | 26 |
| Moderate | 26 |
| Moderate to severe | 5 |
| Severe | 3 |
| PE (yes) | 10 |
| Aortic insufficiency | |
| Normal | 4 |
| Trivial | 7 |
| Mild | 22 |
| Mild to moderate | 12 |
| Moderate | 49 |
| Moderate to severe | 3 |
| Severe | 3 |
| Aortic valve | |
| Tricuspid | 86 |
| Bicuspid | 14 |
| Tricuspid regurgitation | |
| Normal | 6 |
| Trivial | 5 |
| Mild | 39 |
| Mild to moderate | 20 |
| Moderate | 26 |
| Moderate to severe | 2 |
| Severe | 2 |
| AVA (cm2) | 0.72 ± 0.17 |
| EF (%) | 51.7 ± 9.04 |
| MPG (mmHg) | 49.29 ± 15.43 |
| PPG (mmHg) | 81.86 ± 23.4 |
| sPAP (mmHg) | 39.49 ± 11.59 |
Abbreviations: LV, left ventricular; LVE, left ventricular enlargement; RV, right ventricular; RVE, right ventricular enlargement; PE, pleural effusion; AVA, aortic valve area; EF, ejection fraction; MPG, mean pressure gradient; PPG, peak pressure gradient; sPAP, systolic pulmonary artery pressure.
a Values are expressed as mean ± SD.
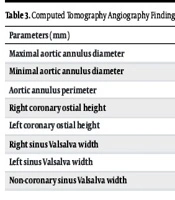
As shown in Table 3, the CT angiography findings of patients before the procedure highlight key measurements related to the aortic anatomy. The maximal aortic annulus diameter averaged 26.53 ± 2.78 mm, while the minimal diameter was slightly smaller at 22.01 ± 2.06 mm. The aortic annulus perimeter measured at 76.76 ± 7.84 mm, indicating the overall circumference of this critical structure. The heights of the coronary ostia showed variability, with the right coronary ostial height averaging 14.12 ± 2.95 mm and the left coronary ostial height at 13.1 ± 2.66 mm. Additionally, diameters of the sinus Valsalva regions were recorded, with the right sinus measuring 29.46 ± 3.73 mm, the left sinus at 30.57 ± 3.96 mm, and the non-coronary sinus at 31.32 ± 3.98 mm.
| Parameters (mm) | Values |
|---|---|
| Maximal aortic annulus diameter | 26.53 ± 2.78 |
| Minimal aortic annulus diameter | 22.01 ± 2.06 |
| Aortic annulus perimeter | 76.76 ± 7.84 |
| Right coronary ostial height | 14.12 ± 2.95 |
| Left coronary ostial height | 13.1 ± 2.66 |
| Right sinus Valsalva width | 29.46 ± 3.73 |
| Left sinus Valsalva width | 30.57 ± 3.96 |
| Non-coronary sinus Valsalva width | 31.32 ± 3.98 |
a Values are expressed as mean ± SD.
Based on the procedural results presented in Table 4, vascular access was achieved via the femoral route in all cases, with valves well-seated in 99% of participants. Procedural complications occurred in 12% of patients and included tamponade, hematoma, ventricular tachycardia (VT), mild PVL, transient atrial fibrillation (AF), and gastrointestinal bleeding (GIB), among others. Notably, one patient died during the procedure due to aortic annular rupture. The procedural mortality rate was 1%.
| Parameters | Values |
|---|---|
| Vascular access (femoral) | 100 |
| Procedural complication (yes) | 12 |
| Procedural mortality (yes) | 1 |
| Post-procedural para valvular leakage | |
| Normal | 67 |
| Trivial | 5 |
| Mild | 25 |
| Mild to moderate | 2 |
| Moderate | 1 |
| Well-seated (yes) | 99 |
| Post-procedural MPG (mmHg) | 9.75 ± 6.19 |
| Post-procedural PPG (mmHg) | 19.25 ± 10.15 |
| ΔMPG (mmHg) | 62.61 ± 24.75 |
| ΔPPG (mmHg) | 39.54 ± 16.55 |
| 1 month mortality | 3 |
| 1 - 6 months mortality | 6 |
| 6 months of all-cause hospitalization | 23 |
Abbreviations: MPG, mean pressure gradient; PPG, peak pressure gradient.
a Values are expressed as mean ± SD.
Post-procedural evaluations revealed a MPG of 9.75 ± 6.19 mmHg and a PPG of 19.25 ± 10.15 mmHg. The changes in MPG (ΔMPG) and PPG (ΔPPG), indicating the reduction in gradients, were 62.61 ± 24.75 mmHg and 39.54 ± 16.55 mmHg, respectively. The PVL assessments indicated normal findings in 67% of cases, with trivial leakage in 5%, mild in 25%, mild to moderate in 2%, and moderate in 1%.
Regarding subsequent hospitalization, patients experienced various issues, including cardiovascular complications, valve thrombosis, arrhythmias, pericardial effusion, myocarditis, coronary artery bypass graft, dyspnea, and cerebrovascular accidents (CVA). Mortality rates reflected ongoing challenges; within one month, there was a 3% mortality rate, attributed to severe and acute femoral artery stenosis leading to disseminated intravascular coagulation (DIC) and necessitating peripheral vascular surgery. In this period, thrombosis was linked to the improper use of anticoagulant medication. Between one and six months, the mortality increased to 6%, with causes including liver cirrhosis, septicemia, and stroke. Moreover, 23% of patients experienced all-cause hospitalization within six months. These findings highlight procedural safety and early clinical outcomes, emphasizing the importance of careful post-procedural management.
4.2. Association of Improvement with Patient Baseline Parameters
The patient improvement was evaluated through ΔMPG and ΔPPG, and the occurrence of procedural complications and PVL, the most common post-procedural complication. The objective was to identify potential factors influencing patient improvement.
The analysis revealed no significant association between ΔMPG, ΔPPG, and the presence or absence of PVL concerning valve type (bicuspid or tricuspid), with P-values of 0.14, 0.47, and 0.99, respectively. In contrast, both ΔMPG and ΔPPG exhibited a significant inverse correlation with the AVA (r = 0.42, P = 0.0001 and r = 0.44, P = 0.0001, for ΔMPG and ΔPPG respectively). Additionally, a direct and significant relationship was found between EF with ΔMPG (r = 0.54, P = 0.0001) and ΔPPG (r = 0.43, P = 0.0001).
Furthermore, when evaluating the relationship between baseline MPG and PPG values before the procedure and the occurrence of PVL post-procedure, it was observed that only a higher baseline MPG was significantly associated with the occurrence of PVL (P = 0.0001), while AVA (P = 0.5) and PPG (P = 0.2) showed no significant correlation.
Finally, an assessment of the relationship between the occurrence of procedural complications and the calculated STS score before the procedure indicated that a higher STS score was significantly correlated with the occurrence of complications (P = 0.03).
5. Discussion
In this study, the relationship between patient improvement, measured through ΔMPG and ΔPPG, and the occurrence of procedural complications and PVL (the most common post-procedural complication) was evaluated in conjunction with various parameters. This investigation aimed to detect potential indexes that determine the level of patient improvement. While TAVI is a well-established procedure for symptomatic AS in elderly and moderate-to-high-risk patients (average age 77, average STS score 7), this study contributes uniquely to the global TAVI literature by reporting outcomes from a tertiary center in Iran, a region with limited data on TAVI safety and efficacy. This regional perspective provides valuable insights into procedural outcomes in a Middle Eastern population, where healthcare infrastructure and patient demographics may differ from Western cohorts.
Before presenting the analytical results, we will discuss the frequency of mortality and morbidity in our study and compare them with other studies to highlight that our complication and mortality rates are acceptable. In a meta-analysis, it was reported that at 30 days, 7.5% of patients died (22). Another study reported that the all-cause 30-day mortality rate was 8.7% (n = 19) (23). In our study, the 30-day mortality rate was 2.5%, which is notably lower than these reported rates, suggesting that our outcomes are within acceptable ranges. This finding is particularly significant in the context of a developing healthcare system, demonstrating the feasibility of achieving favorable TAVI outcomes in resource-constrained settings.
To further contextualize our outcomes, Table 5 compares our study’s mortality and complication rates with those from contemporary TAVI trials and registries, including the PARTNER 3 trial (2.6% 30-day mortality in low-risk patients), SURTAVI (5% in intermediate-risk patients), and BASILICA (7% in high-risk patients) (24-28). Our study’s procedural complication rate of 12%, procedural mortality of 1%, 30-day mortality of 3%, and 6-month mortality of 6% in a moderate-to-high-risk cohort (mean STS score 7.22, age 77.82 years, 55% male) are comparable to or lower than these benchmarks, particularly for high-risk populations (BASILICA). This suggests robust procedural safety in our tertiary center in Iran, despite a developing healthcare infrastructure. Consistent with prior literature, the EuroSCORE was less reliable for risk stratification in our TAVI population, reinforcing our reliance on the STS score to predict procedural complications (P = 0.03).
| Studies (Y) | Sample Size | Mortality Rate (%) | Age Range | Male (%) | STS Score (Mean) | Patients’ Characteristics |
|---|---|---|---|---|---|---|
| PARTNER 3 (2020) | 1000 | 2.6 | 65 - 85 | 58 | 1.9 | Low-risk patients |
| SURTAVI (2018, extended 2019 update) | 1100 | 5 | 70 - 85 | 63 | 5.1 | Intermediate-risk patients |
| FORCE trial (2021) | 500 | 6.4 | 70 - 85 | 60 | 5.6 | Intermediate-high risk |
| STACCATO registry (2022) | 2200 | 6.4 | 75 - 90 | 62 | 6.3 | Real-world data, mixed risk profiles |
| BASILICA trial (2022) | 500 | 7 | 75 - 85 | 65 | 7.1 | High-risk patients |
Abbreviation: STS, society of thoracic surgeons.
In the current study, we investigated the relationship between patient improvement, as measured by ΔMPG and ΔPPG, and the occurrence of procedural complications, including PVL, which is recognized as the most common post-procedural complication. Vascular complications (VCs) occurred in approximately 28.7% of cases, with 3.38% classified as major. The majority of these incidents (10.9%) took place during the operation, with 1.6% being serious in nature and the remainder minor. The postoperative period saw 17.2% of VCs, with only 1.3% being categorized as major. In some instances, procedures were required to rectify these complications, with 4.2% of cases necessitating intervention. Notably, dissections and hematomas comprised the largest share of these incidents.
At 30 days following the procedure, 2.5% of patients had passed away, with a significant correlation established between peri-operative VCs and mortality (P < 0.001). Additionally, 3.8% of patients were readmitted within 30 days, with a small percentage (1.3%) related to VCs, including hematoma and infection (29). These findings underscore the importance of addressing VCs in the context of this medical procedure. As a general rule, 3 - 4% procedural mortality is acceptable.
Our analysis revealed that there was no significant correlation between ΔMPG, ΔPPG, and the presence or absence of PVL with valve type, whether it be a bicuspid or tricuspid configuration. PVL is a common complication following TAVI in patients with a bicuspid aortic valve (BAV). However, the impact of valve type on the degree of gradient improvement among patients has not been extensively investigated. In our cohort, PVL occurred in 33% of patients (25% mild, 2% mild-moderate, 1% moderate, Table 4), which is comparable to contemporary studies reporting 20–30% mild PVL with newer-generation balloon-expandable valves (30). The lack of association between PVL and valve type (86% tricuspid vs. 14% bicuspid, P = 0.99) aligns with a comprehensive review of 2,394 patients undergoing tricuspid or bicuspid TAVR, which found no significant differences in PVL incidence (30). These findings suggest that the newer valve devices yield comparable outcomes regardless of the aortic valve type, indicating the need for further research to elucidate these relationships and enhance our understanding of the implications for patient management.
ΔMPG and ΔPPG showed a significant inverse correlation with AVA. Therefore, in the present study, the improvement in gradient was less evident at low EFs. As expected, a smaller baseline AVA, indicative of more severe AS, was associated with greater reductions in MPG and PPG post-TAVI (r = 0.42, P = 0.0001 for ΔMPG; r = 0.44, P = 0.0001 for ΔPPG), quantifying the strength of this intuitive relationship and reinforcing baseline AVA as a key predictor of hemodynamic improvement. However, this correlation is not the sole determinant of outcome, as other factors, such as LVEF, also significantly influence gradient improvement (r = 0.54, P = 0.0001 for ΔMPG; r = 0.43, P = 0.0001 for ΔPPG). This correlation between gradient improvement and baseline parameters like AVA and LVEF provides critical insights into predictors of procedural success, which can guide patient selection and risk stratification in similar populations.
Our investigation demonstrated a significant and direct relationship between ΔMPG and ΔPPG and LVEF. In patients undergoing TAVI for AS, existing studies suggest that reduced LVEF and low AVG are associated with inferior long-term outcomes. However, due to their coexistence, the degree to which these factors independently contribute to outcomes after TAVI remains unclear (31). The observed correlations between LVEF and gradient improvement (r = 0.54, P = 0.0001 for ΔMPG; r = 0.43, P = 0.0001 for ΔPPG) reflect associations rather than causation, and their predictive power may be limited by confounding factors such as myocardial fibrosis.
Previous research highlights the prevalence of myocardial fibrosis in low-flow, low-gradient AS, characterized by abnormal LV remodeling, reduced LV compliance, and diminished filling capacity, which can impair LVEF’s prognostic utility (32-34). This condition has been linked to poorer clinical outcomes in severe AS (35). Conversely, reduced LVEF in these patients may indicate irreversible myocardial dysfunction or afterload mismatch resulting from valvular obstruction. In patients with preserved resting aortic valve gradient, afterload mismatch due to valvular obstruction is likely to improve significantly following AVR, whether surgical or transcatheter (36, 37).
To better account for confounders like myocardial fibrosis, future studies should incorporate advanced imaging, such as cardiac magnetic resonance imaging (MRI), to quantify fibrosis and refine the prognostic role of LVEF in TAVI outcomes. Our results corroborate this finding, underscoring the importance of afterload in contributing to LV function and outcomes in patients with AS undergoing TAVI.
Also, in examining the relationship between the initial MPG, PPG, and AVA values before the procedure and the occurrence or absence of PVL after TAVI, it was observed that only MPG was more associated with the occurrence of PVL. Regarding the association of high MPG with the presence of PVL and the lack of association between PPG and AVA, it has been suggested that PPG is a point measurement, whereas MPG calculates the logarithm of the measured gradient points and provides a more general picture of the gradient status. Therefore, it can represent the status with a better approximation. The AVA measurement is also very operator-dependent, and in our study, the operators were different.
The STS risk model, utilized to predict operative mortality in cardiac surgery, is frequently applied in the risk assessment of patients considered for TAVI. Our examination of the relationship between procedural complications and the pre-TAVI STS score revealed a significant correlation, indicating that higher STS scores were associated with an increased incidence of complications. Additionally, we noted that patients who developed procedural complications had higher STS scores. This finding is not unexpected, as the STS score accounts for various factors, including demographics, laboratory results, medication usage, comorbidities, and cardiac status. While prior studies have predominantly assessed mortality related to TAVI using the STS score, our investigation specifically focused on procedural complications in relation to the STS score.
The 30-day clinical outcomes stratified by STS score have been explored in several previous reports (38, 39). Consistent with these studies, our findings indicated that the STS score overestimated 30-day mortality. The results imply that not only high-risk patients but also low-risk patients — who were selected for TAVI due to other comorbidities, such as frailty, cancer, and pre-operative conditions related to non-cardiac surgeries — benefited from the procedure. Furthermore, the shift from general anesthesia to conscious sedation in the last three years of our study reflects an adaptation in TAVI protocols that may be particularly relevant for resource-constrained settings, offering practical insights for procedural planning in similar healthcare systems.
Regarding procedural outcomes, complications arising from anatomical or technical factors, including coronary obstruction, cardiac tamponade, conversion to open surgery, and VCs, were reported to be comparable regardless of the STS score (31). However, in contrast to previous reports on earlier-generation devices (40), our study demonstrated that VCs and related bleeding events were less prevalent and comparable among low- and intermediate-risk patients in comparison to high-risk patients.
Few prior studies have investigated the association between STS score and long-term clinical outcomes following TAVI; however, two studies have indicated that a high STS score was linked to worse long-term prognoses after the implantation of earlier-generation self-expandable valves (40, 41).
5.1. Conclusions
In conclusion, this study highlights the demographic and clinical characteristics of a patient cohort undergoing TAVI, revealing a moderate risk profile characterized by various comorbidities and procedural outcomes. The findings underscore the importance of monitoring post-procedural complications, such as PVL and vascular issues, which can significantly impact patient recovery and mortality rates. Moreover, the inverse relationship between improvement parameters and the AVA suggests a critical link between cardiac function and procedural success. Overall, these results emphasize the need for tailored patient management strategies to enhance outcomes in individuals with AS. Future research should focus on further elucidating the factors affecting long-term outcomes in this patient population.
5.2. Limitations
As with any investigation, this study had its own limitations. Due to resource constraints at our center, alternative risk assessment tools, such as frailty indices (e.g., gait speed, albumin levels), were not collected, limiting the comprehensiveness of our risk stratification beyond the STS score. The restriction to a 6-month follow-up, due to the retrospective study design and the completion of data collection at submission, limits the evaluation of TAVI durability. Future prospective studies should prioritize collecting 1-year follow-up data to assess long-term outcomes.