1. Background
The thyroid's historical origins trace back to 2697 BCE, marked by the "Yellow Emperor" Hung Ti's mention of seaweed's therapeutic use for goiter. The term "thyroid" was introduced by Thomas Wharton in 1656 and is derived from the Greek word for shield. In 1811, Bernard Courtois identified iodine upon observing a distinct purplish residue during the combustion of seaweed (1). Around a century ago, the exploration and study of iodine as an essential constituent of thyroid hormone (TH), as well as iodination as a preventive measure against goiter, were undertaken (2).
Iodine is an essential element carried to the body through dietary intake. As a critical nutrient, iodine is important in synthesizing TH (3). The majority of iodine content in the human body is found in the thyroid gland, where approximately 70 - 80% of the total amount of 15 - 20 mg of iodine in a healthy adult's body is stored (4). The insufficient intake of iodine has repercussions on the physical and mental development of millions of individuals across the globe (5). The issue of iodine deficiency (ID) was focused on endemic goiter (6). The sources of iodine are vegetables grown in iodine-rich soil, drinking water, salt, and other seafood. Iodine trapping is the initial stage of iodine metabolism. This process begins with the transport of iodine from the capillaries to the follicular cells of the thyroid gland via an active transport system that occurs due to the chemical and electrical gradients of sodium/iodine symporter proteins on the basolateral membrane of the follicular cells (7).
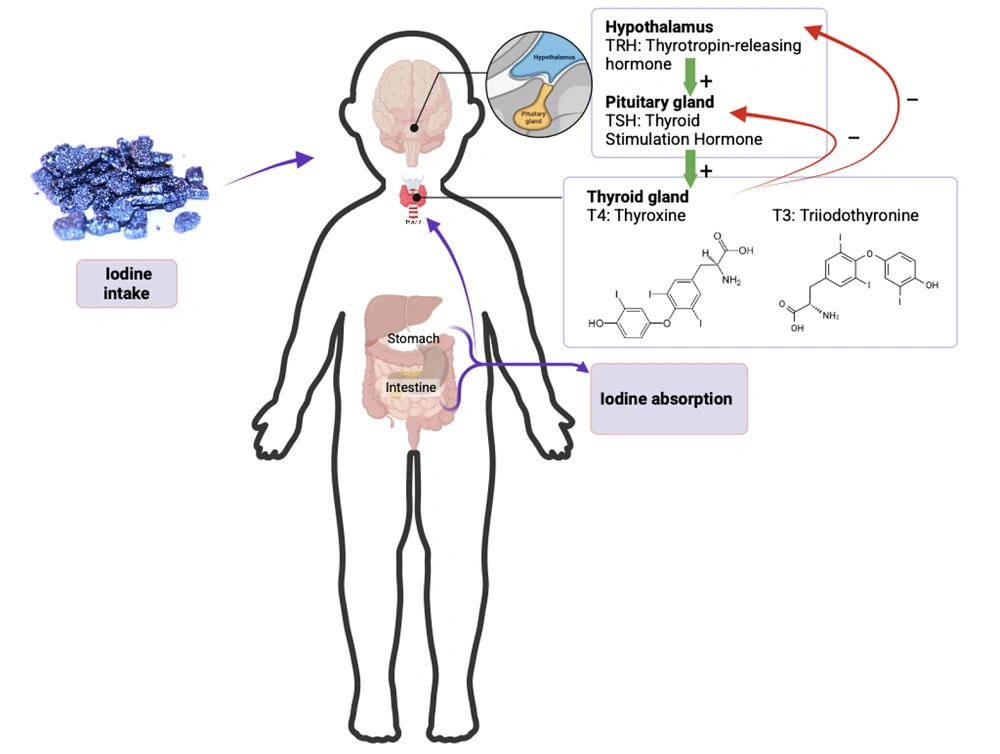
The pituitary gland regulates TH through thyroid stimulating hormone (TSH), which operates via a feedback mechanism with T4 in the blood (Figure 1). A decrease in T4 will stimulate the pituitary gland to produce TSH, thereby increasing the release of T4 from the thyroid and maintaining TH levels (7). T4 is the main form of TH circulating in the blood. Testing for free T4 (FT4) is necessary to measure the amount of T4 that is unbound and working in tissues. Assessing FT4 and TSH is more accurate in evaluating thyroid function. Any changes in the TH axis are first evident in FT4 levels (4, 7). The production of THs T3 and T4 is influenced by TSH released from the anterior pituitary gland, which is regulated by thyrotropin-releasing hormone (TRH) from the hypothalamus. Positive and negative feedback mechanisms in the hypothalamic-pituitary-thyroid axis aim to maintain TH levels within normal limits. Decreased TSH levels lead to a decline in the output of THs, specifically T3 and T4. On the other hand, decreased levels of T3 and T4 will trigger the secretion of TSH (8). The development of the screening method for hypothyroidism in newborns, which is a global initiative aimed at early detection and commencement of treatment to prevent intellectual disability and mortality, was attributed to Robert Guthrie in 1963 (9).
Short stature is one of the leading health issues facing children in Indonesia. Almost 1 in 3 toddlers in Indonesia experiences short stature (10). Children under the age of five in Indonesia are required to undergo regular health check-ups according to the schedule outlined in the well-child visit program at community health centers. During these visits, they will be weighed, measured for height and head circumference, and receive vaccinations. This program also provides educational sessions for mothers on childcare practices, nutrition, and maternal health (11).
2. Objectives
Our research aims to examine the iodine consumption of children living in West Java, analyzing its relationship with FT4 and TSH levels, while also investigating its geographic mapping.
3. Methods
Two hundred and twenty-five healthy children aged 2 to 5 years during well-child visits in 12 primary healthcare centers across 11 subdistricts of Bandung District were included. Exclusion criteria included parents refusing to participate or follow procedures, as well as children with syndromes or a history of chronic underlying diseases such as lupus, chronic kidney disease, or hormonal imbalance.
A cross-sectional study was conducted in 2019 at 12 primary healthcare centers across 11 subdistricts of Bandung District, West Java, Indonesia. The variables measured included weight and height, indicators of children's growth, and nutritional status. Each child's height was then plotted on the World Health Organization (WHO) growth chart Z-score height to age and Body Mass Index (BMI) to age. Children's nutritional status was also determined using the WHO growth chart.
Assessing iodine status in children aged under five years old based on WHO recommendations using urinary iodine concentration (UIC) as the gold standard was challenging. Due to logistical and ethical constraints, we were unable to collect urine samples for UIC analysis. Collecting urine samples from children aged 2 - 5 years is particularly challenging due to toilet training status, potential contamination, and practical limitations in field settings. As an alternative, children's iodine intake was estimated using the 24-hour dietary recall method conducted through interviews with their parents. Professional nutritionists conducted the analysis and recall of food intake. Subsequently, iodine intake was analyzed using the Nutrisurvey program. Dietary intake of iodine has been measured using Semi-quantitative Food Frequency Questionnaire (SQFF), and this method can be used to classify subjects into low and high iodine intake groups (12). Reference values used in our study are as follows: Recommended iodine intake is 30 µg/kg/24 hours for infants, 90 - 120 µg/kg/24 hours for children, and 150 µg/kg/24 hours for adolescents and adults (13).
In this study, blood samples were also collected from each child. To assess TSH levels and FT4 levels, the samples were processed in the clinical pathology laboratory at Hasan Sadikin General Hospital. The TSH levels and FT4 were measured using the Enzyme-Linked Immunosorbent Assay (ELISA) kits from Centaur (14, 15). The reference values used in the present study were determined based on the method used for each kit as follows: Limits of normal TSH for children aged 24 - 59 months: 0.35 - 4.5 mIU/mL; normal FT4 levels for children aged 24 - 59 months: 0.89 - 1.76 ng/dL.
3.1. Statistical Analyses
All baseline demographic and clinical variables are presented as percentages. Correlation in characteristics was analyzed using Pearson. A P-value of less than 0.05 was considered statistically significant. All analyses used IBM SPSS Statistics (Version 26; SPSS Inc., Chicago, IL).
3.2. Ethics
This study was approved by the Health Research Ethics Committee of the Faculty of Medicine, Universitas Padjadjaran Ethical Exemption No. 375/UN6.KEP/EC/2021 for studies involving humans and conformed to the ethical guidelines of the Declaration of Helsinki.
4. Results
Anthropometric measurements, nutritional status, and levels of iodine intake, TSH, and FT4 are included in Table 1. Based on the recommended iodine intake for children aged 2 - 5 years, two subjects had adequate iodine intake, while the rest had inadequate iodine intake. Based on the normal range of TSH levels, all subjects are within the normal range. However, based on the normal range of FT4 levels, 40.44% of subjects have low levels of FT4 (Figure 2).
| Characteristics (N = 225) | Values |
|---|---|
| Age | 43.39 ± 10.19 |
| Gender | |
| Boy | 107 (47.5) |
| Girl | 118 (52.4) |
| Anthropometric indices | |
| Weight (kg) | 12.24 ± 2.13 |
| Height (cm) | 89.4 ± 6.32 |
| Birth weight (kg) | 3.0 ± 0.47 |
| Birth height (cm) | 48 ± 2.61 |
| Height/age | |
| < -3 SD (severely stunted) | 85 (37.78) |
| < -2 SD (stunted) | 74 (32.89) |
| 2 SD ≤ H/A ≥ -2 SD (normal) | 65 (29.33) |
| > 3 SD (very tall) | 0 |
| BMI/age | |
| < -3 SD (severely wasted) | 4 (1.78) |
| < -2 SD (wasted) | 10 (4,44) |
| 2 SD ≤ BMI/U ≥ -2SD | 206 (91.56) |
| > 2 SD (overweight) | 2 (0.89) |
| > 3 SD (obese) | 3 (1.33) |
| Iodine intake | 11.65 ± 17.32 |
| TSH level | 2.69 ± 0.70 |
| FT4 level | 0.98 ± 0.42 |
Main Characteristics
Classification of iodine intake, thyroid stimulating hormone (TSH) levels, and free T4 (FT4) levels based on normal ranges in children aged 2 - 5 years [the recommended iodine intake is 90 - 120 ug/kg/24 hours for children aged 2 - 5 years; normal range of TSH (0.55 - 4.78 µIU/mL) and FT4 (0.89 - 1.76 ng/dL) levels; n = 225.14].
Table 2 reveals a significant correlation between iodine intake, TSH levels, and FT4 levels. In this study, Pearson correlation analysis revealed a statistically significant negative correlation between TSH and iodine intake (R = -0.229, P = 0.036), indicating that children with lower iodine intake tend to have higher TSH levels, despite TSH values remaining within the clinically normal range. This may reflect a subclinical compensatory mechanism due to mild ID. Additionally, a significant inverse correlation was observed between TSH and FT4 levels (R = -0.215, P = 0.049), and a positive correlation between FT4 and iodine intake (R = 0.412, P < 0.001), supporting the role of iodine intake in influencing TH regulation even within normal biochemical ranges.
| Variable, R (Pv) | TSH | FT4 | Iodine |
|---|---|---|---|
| TSH | 1 | -0.215 (0.049) | -0.229 (0.036) |
| FT4 | -0.215 (0.049) | 1 | 0.412 (< 0.001) |
| Iodine | -0.229 (0.036) | 0.412 (< 0.001) | 1 |
Correlation Between Thyroid Stimulating Hormone Levels, Free T4 Levels, and Iodine Intake a
Figure 3 reveals a significant correlation between iodine intake, TSH levels, and FT4 levels. Iodine intake correlates positively with FT4 levels (Figure 3A). The TSH levels correlate negatively with FT4 levels (Figure 3B), indicating that higher TSH levels are associated with lower FT4 levels. Similarly, there is a correlation between TSH levels and iodine intake (Figure 3C). The TSH levels correlate negatively with iodine intake, indicating that higher TSH levels are associated with lower iodine intake.
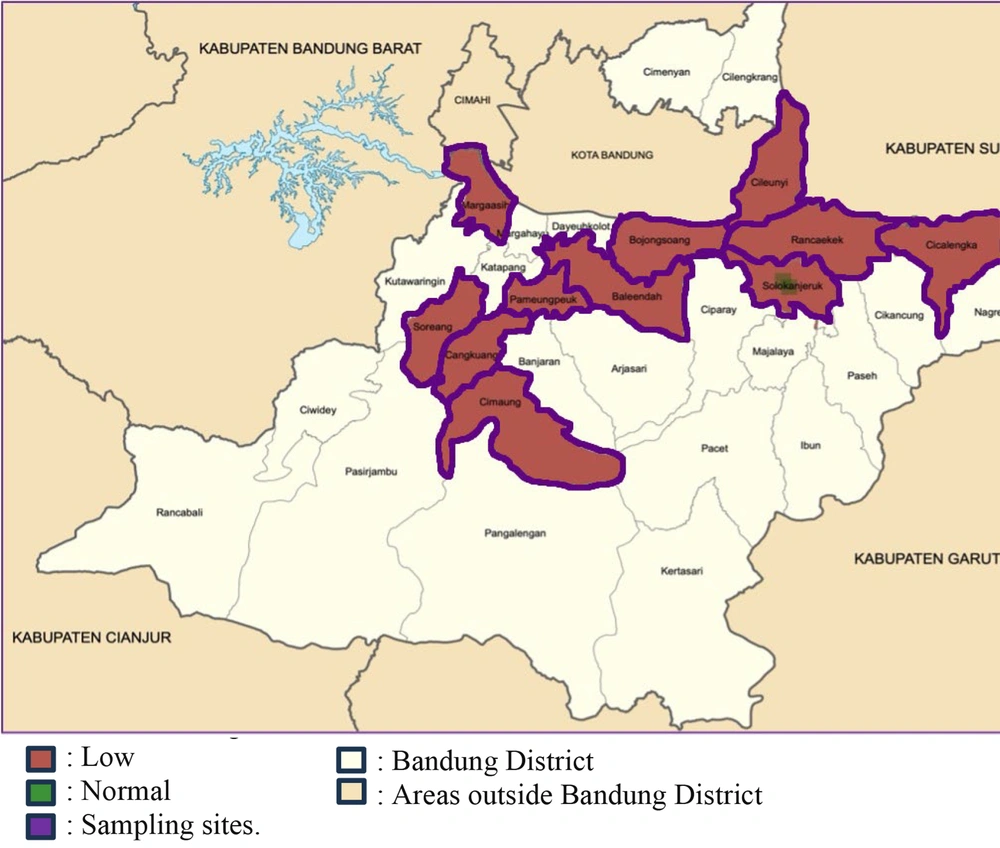
Almost all children in the sampling sites do not have adequate iodine intake (Figure 4). The geographic locations of iodine intake are mapped in 11 subdistricts of Bandung District in West Java.
Geographic mapping of children’s iodine intake (modified from Bandung District Government Website) (15).
5. Discussion
Geographic mapping shows that most children aged 2 - 5 years living in Bandung District, West Java, Indonesia, have inadequate iodine intake (Figure 4). Iodine, as an important and singular substance for producing TH, has been highlighted in this study. Additionally, a significant correlation has been uncovered between low iodine and low FT4, linked to high TSH.
This result is consistent with the 2013 Basic Health Research conducted by the Health Research and Development Agency of the Ministry of Health of the Republic of Indonesia across 34 provinces, which indicates that West Java is ranked as the eighth lowest province in terms of iodine-sufficient salt consumption. The prevalence of salt consumption without iodine in West Java province is 10.9%. Moreover, only 68.6% of the total population in West Java consumes iodized salt in adequate amounts, leaving the remaining 20.5% classified as deficient (16).
The study by Simbolon and Hapsari conducted in Bengkulu, a coastal area, found that despite this, 71.2% of children under five years old did not have sufficient iodine consumption (17). Amalia et al.’s research indicates that a significant portion of children in Indonesia require adequate iodine intake, with less than 77% meeting the recommended levels. Only a quarter of the salt available in Indonesia contains sufficient iodine content according to Indonesian standards [Standar Nasional Indonesia (SNI)], which is 30 - 80 ppm. The majority, comprising 75%, have less than 30 ppm (18).
Iodine deficiency disorder (IDD) encompasses both clinical and subclinical effects of inadequate iodine levels. Given iodine's essential role in synthesizing T3 and T4 hormones, its scarcity significantly disrupts hormone production. Initially, the thyroid gland responds by releasing hormones stored within thyroglobulin molecules. However, as iodine stores deplete and the blood level of T4 decreases, the pituitary gland increases TSH output. Consequently, persistent TSH stimulation in endemic regions triggers thyroid gland hypertrophy and follicular cell hyperplasia, resulting in goiter formation and potential enlargement to considerable proportions (4).
Dalili et al. emphasized the importance of using indicators recommended by WHO, including UIC and neonatal TSH screening, in assessing ID across different populations. He compared IDD using two WHO-recommended indicators: Serum TSH levels in newborns and UIC in school-aged children across two populations in Iran. Their findings showed a non-endemic IDD status despite a higher incidence of congenital hypothyroidism (CH), suggesting that CH in this region may not be caused by ID (19).
Bandung District features diverse geographical conditions, situated in a highland region characterized by hills and mountains. This topographical profile may contribute to limited access to adequately iodized salt, especially in remote or hard-to-reach areas. In line with this, data from West Java province indicate that 10.9% of the population consumes salt without iodine (16), highlighting a significant public health concern, particularly for vulnerable groups such as young children.
Although UIC is the gold standard for assessing iodine status, as recommended by the WHO, our study relied on dietary iodine estimates using validated 24-hour recall and SQFF methods due to logistical and ethical challenges in collecting urine samples from young children (19). Despite this limitation, the observed physiological changes mirror expected thyroid responses to ID. These responses are consistent with the feedback mechanisms described in iodine-deficient states, where inadequate iodine intake leads to decreased FT4 (7, 8).
Although all subjects in this study had TSH levels within the normal reference range, it is notable that 40.44% of them exhibited low levels of FT4. This finding suggests the presence of subclinical or early-stage thyroid dysfunction, where FT4 levels begin to decline despite TSH remaining within normal limits. This may occur in the early compensatory phase of ID, where the thyroid is still responding to regulatory signals but hormone synthesis is already impaired due to insufficient iodine availability (20).
Most of the children in this study have short stature with a normal BMI, despite being mostly born with normal weight and length. During well-child visits, all of the subjects have no complaints and no history of diseases that could disturb their growth. This phenomenon is in line with a study by Novina et al., which revealed that a significant number of children under five years old living in West Java were classified as stunted (56.31%) while having a normal BMI (89.92%), based on the 2006 WHO Growth Chart Standards (21). Regarding low iodine intake based on SQFF methods and stature, Simbolon et al. reported that almost all of the children with short stature in Bengkulu, Indonesia, have low iodine intake (17). Low FT4 levels were observed in short stature adolescents by Gutch et al. (22). Dhanjal and Singh also reported decreased levels of FT3, FT4, and TSH were found in undernourished and short stature toddlers (23). Shaheen noted that FT4 and TSH levels are decreased in malnourished children compared to those without undernutrition (24). Adamczewska indicated that normal high TSH levels without disruptions in FT4 levels are linked to lower levels of GH and insulin-like growth factor 1 (IGF-1) in the evening, resulting in shorter stature in children (25).
The TH plays a significant role in childhood growth alongside other growth factors such as growth hormone, IGF-1, insulin, sex steroids, and leptin. In peripheral tissues, thyroxine undergoes conversion into triiodothyronine, which is typically regarded as the hormone with physiological activity. The presence of thyroid hormone receptors α1 (TRα1) and thyroid hormone receptors β1 (TRβ1) has been demonstrated in both the resting and proliferative regions of the growth plate. Local regulation encompasses processes such as chondrocyte maturation, synthesis of cartilage matrix, mineralization, and degradation. Hypothyroidism occurring during childhood and adolescence leads to a delay in skeletal maturation and impedes growth (26).
This study has several limitations. First, we did not measure UIC, which is the WHO-recommended gold standard for assessing iodine status. Instead, iodine intake was estimated using dietary recall methods, which, while validated, are subject to recall bias and may not fully reflect actual iodine status. Second, the cross-sectional design of the study limits our ability to establish causal relationships between iodine intake and TH levels. Third, urine sampling and longitudinal follow-up were not feasible due to ethical and logistical constraints, especially in children aged 2 - 5 years. Finally, the study was geographically limited to Bandung District, West Java, which may affect the generalizability of the findings to other regions in Indonesia with different iodine availability or dietary patterns.
5.1. Conclusions
The study reveals that almost all children living in Bandung District, West Java, Indonesia, have low iodine intake. This finding is concerning as iodine is essential for the synthesis of THs, which play crucial roles in various physiological processes. Low iodine intake can affect TSH and FT4 levels in the body. These THs influence a range of bodily functions, including metabolism, growth, and development. Future research is recommended to examine the broader clinical impacts of ID, particularly its association with goiter development, growth retardation, and cognitive outcomes in children. Longitudinal studies incorporating clinical thyroid assessments, growth monitoring, and cognitive testing will be essential to provide a more comprehensive understanding of ID and its long-term effects on child health and development.
![Classification of iodine intake, thyroid stimulating hormone (TSH) levels, and free T4 (FT4) levels based on normal ranges in children aged 2 - 5 years [the recommended iodine intake is 90 - 120 ug/kg/24 hours for children aged 2 - 5 years; normal range of TSH (0.55 - 4.78 µIU/mL) and FT4 (0.89 - 1.76 ng/dL) levels; n = 225.14]. Classification of iodine intake, thyroid stimulating hormone (TSH) levels, and free T4 (FT4) levels based on normal ranges in children aged 2 - 5 years [the recommended iodine intake is 90 - 120 ug/kg/24 hours for children aged 2 - 5 years; normal range of TSH (0.55 - 4.78 µIU/mL) and FT4 (0.89 - 1.76 ng/dL) levels; n = 225.14].](https://services.brieflands.com/cdn/serve/3170c/0bb4784143cb9ae32c2feeddbe9c47c82b2f78af/jcp-16-4-161273-i001-preview.webp)
![A - C, correlation between thyroid stimulating hormone (TSH) levels, free T4 (FT4) levels, and iodine intake [the values represent Pearson’s correlation coefficient with significance at P < 0.05 (n = 225); abbreviations: R, Pearson correlation coefficient; Pv, P-value]. A - C, correlation between thyroid stimulating hormone (TSH) levels, free T4 (FT4) levels, and iodine intake [the values represent Pearson’s correlation coefficient with significance at P < 0.05 (n = 225); abbreviations: R, Pearson correlation coefficient; Pv, P-value].](https://services.brieflands.com/cdn/serve/3170c/f4cdf6b7779693d3f96b21919c4a0d13d508f50e/jcp-16-4-161273-i002-preview.webp)