1. Background
Doxorubicin (DOX), an anthracycline antibiotic, has been commonly used in treating numerous solid tumors since 1969 (1, 2). However, its effectiveness is limited, particularly by cardiotoxicity (3), which could lead to heart failure and cardiomyopathy within months or even years after treatment discontinuation, especially among younger individuals (1). While the molecular mechanisms underlying cardiotoxicity caused by DOX (CTD) have not been completely elucidated, mitochondrial dysfunction (4) and the subsequent accumulation of dysfunctional and fragmented mitochondria through disruption of the dynamic processes of mitochondrial fission and fusion (under the title "mitochondrial dynamics") is considered the first main step of CTD (2, 3). The DOX could decline the expression of fusion-related proteins: Mitofusion1&2 (MNF1 and MNF2), and optic atrophy 1 (OPA1) (5) and increase the expression of the fission-related protein dynamin-related protein 1 (DRP1), along with fragmentation of mitochondria (5, 6). However, it was stated that DOX could increase the RNA expression of fusion-related proteins (MNF2 and OPA1) while increasing mitochondrial fragmentation (7). Therefore, there are contradictory results regarding proteins related to mitochondrial fusion.
On the other hand, damaged and fragmented mitochondria could be destroyed via the autophagy process (8), but previous studies show conflicting results about the effect of DOX on the autophagy process (5, 9). It has been reported that DOX could lead to the enhancement of autophagy (6) by increasing the gene expression of autophagy-related proteins [microtubule-associated protein light chain 3 (LC3), Beclin1, PTEN-induced putative kinase 1(Pink1), Parkin, and p62] (5). Despite this, Pizarro et al. showed that DOX could inhibit the autophagy process by reducing the expression of Beclin1 levels and stimulating the protein kinase B (PKB), also known as Akt (Akt)/ the mammalian target of rapamycin (mTOR) signaling pathway (9). Therefore, more research is needed in this area.
Due to the utility of DOX in cancer treatment, moderate-intensity endurance training (MCT) has been considered an important non-pharmacological strategy to deal with CTD (4, 10). On the other hand, HIIT, which includes repeated sets of high-intensity exercise with moderate-to-low-intensity active recovery periods, induces greater cardiovascular adaptations than MCT (11, 12). There is strong evidence that the increased cellular stress in HIIT, compared to MCT, results in greater mitochondrial adaptation (13). Recent research has also shown that HIIT could reduce CTD by reducing heart damage biomarkers, including levels of lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB) (14), and increasing the amount of antioxidant enzymes in the heart tissues (15). Also, HIIT could reduce CTD by increasing the expression of mitophagy genes (Parkin and Mfn2) (16).
2. Objectives
To our knowledge, limited studies have investigated the preventive role of HIIT against CTD by focusing on mitochondrial dynamics and the autophagy process. Therefore, this research aimed to investigate the preventive effects of HIIT on CTD by examining the expression levels of genes associated with autophagy, specifically Beclin1 and LC3II, as well as genes related to mitochondrial dynamics, including FIS1, DRP1, OPA1, and Mfn2, in the left ventricle of rat hearts.
3. Methods
3.1. Animals
Twenty-four male Wistar rats, aged 3 weeks and weighing 250 - 270 g, were obtained from the Research Center of Tabriz University of Medical Sciences. To adapt to the new environment, animals were housed in a temperature-controlled room set at 21 ± 2°C, providing 12 hours of light and 12 hours of darkness, with unrestricted access to food and water for one week. Then, to get familiar with the training, rats were trained on the rodent treadmill for 3 sessions a week, 15 minutes at a speed of 10 m/min for one week. After getting acquainted with the exercise training, the animals were randomly divided into 4 groups (n = 6): Control (control); treatment with DOX (DOX); exercise training (HIIT); and DOX-treatment after 8 weeks of HIIT (HIIT + DOX).
3.2. The High-intensity Interval Training Protocol
The HIIT protocol on the treadmill, which was developed based on Jiang et al.'s research (17), included three stages: (1) Ten minutes of warm-up with an intensity of 50% - 55% maximal oxygen uptake (VO2max), (2) seven consecutive intervals of four minutes of exercise isolated with three minutes of exercise with moderate intensity, respectively, with 80 - 90% and 65-75% VO2max, and (3) one minute of the cooldown period with an approximate intensity of 50 to 55% of VO2max. The animals trained on the rodent treadmill for 60 minutes, 5 days a week for 8 weeks. Despite the lack of direct access to estimate VO2max, the training intensity was estimated indirectly based on recent research by Hoydal et al. (18). Based on this research, after 10 - 20 minutes of warm-up, the treadmill rate sped up every two minutes by 0.03 m/s (1.8 - 2 m/min). The speed at which their VO2max was reached was when they couldn't keep running with the increasing treadmill speed.
3.3. Doxorubicin Injection and Tissue Removal
Immediately following the final HIIT session, the DOX and HIIT + DOX groups received a single cumulative intraperitoneal injection of 20 mg/kg body weight of hydrochloride DOX (19). After 72 hours of DOX injection and an overnight fast, the rats were anesthetized with xylazine (10 mg/kg body weight) and ketamine (90 mg/kg body weight), and then the heart was excised as rapidly as possible. Immediately, all left ventricular tissue samples were transferred to an RNase inhibitor solution (manufactured by Qiagen) and stored at -80°C until RNA extraction (20). Groups without DOX treatment received the same amount of saline.
3.4. RNA Isolation and Complementary DNA Synthesis
The real-time PCR technique was used to evaluate the expression level of OPA1, DRP1, FIS1, Mfn2, LC3II, and Beclin1. In this process, total RNA was carefully isolated from the tissue samples of the left ventricle using TriPure isolation reagent solution (manufactured by Roche) according to the instructions. To determine the quality and quantity of extracted RNAs, the optical density (OD) of all samples was measured with a Thermo Scientific NanoDrop 2000c spectrophotometer device. Then, complementary DNA (cDNA) was synthesized as template DNA using a cDNA synthesis kit (manufactured by TAKARA company) according to the manufacturer's instructions (20).
3.5. Real-time Quantitative Polymerase Chain Reaction Technique
Quantitative analysis was performed using the StepOnePlus Real-time PCR system. The relative expression of OPA1, DRP1, FIS1, Mfn2, LC3II, and Beclin1 in the heart tissue samples was measured using gene-specific primers and Cybergreen Master Mix (manufactured by TAKARA) on the LightCycler 96 Instrument (Bio-Rad). To normalize the expression levels of all target genes, the parallel measurement of the expression level of GAPDH (housekeeping gene) was used. The sequences of the primers used in this study are shown in Table 1. Additionally, according to reference gene expression and Pfaffl's method, target gene expression was measured (21).
| Genes | Melting Temperature (Tm) | Forward | Reverse | Product Length (bp) |
|---|---|---|---|---|
| DRP1 | 61 | GGAGAAAAGGAAGCAAGCGGG | CAATCTGAGGCAGCTGGATGA | 123 |
| OPA1 | 59 | GCTCTATGCCCTTGTTGCTGA | TTGGCAGACTTCACAGGCCAG | 168 |
| Mfn2 | 59 | TGGACCTGAATCGGCACAGAG | ATCGAGAAAAGAGCAGGGACA | 129 |
| FIS1 | 61 | GGGACCCAAGCGTGCTTTCTG | TCTGACAAAGGACAGTCCCAA | 82 |
| Beclin1 | 59 | GTCAGCTCTCGTCAAGGCGTC | CGAGACCTCCAGAGTTCCCAT | 103 |
| LC3II | 61 | GTCCAGGCTAGCGGGGAGATA | AGTAACCTTTAGGGCGGCAG | 83 |
Abbreviations: OPA1, optic atrophy 1; LC3, light chain 3.
3.6. Statistical Analysis
Data are expressed as mean ± standard deviation. Statistical analysis was completed with IBM SPSS software (version 23). The Shapiro-Wilk test was used to check the normality of the data. Additionally, one-way ANOVA ("analysis of variance") was used to analyze the data with Tukey's post hoc test for any comparison between groups (α < 0.05).
4. Results
4.1. Proteins Related to Mitochondrial Dynamics
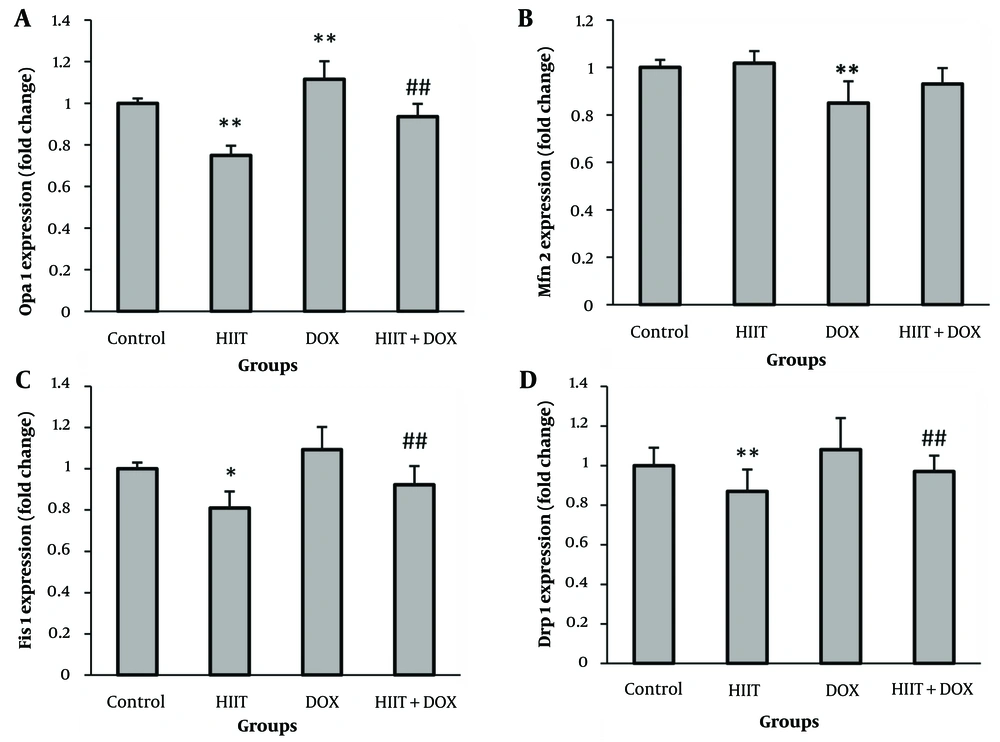
Results showed that DOX induction led to not only a non-significant increase in the FIS1 and DRP1 mRNA levels but also a significant decrease in Mfn2 mRNA levels (P = 0.000) (an important protein mitochondrial fusion controller) compared to the control group. Surprisingly, DOX induction significantly increased OPA1 mRNA level compared to the control group (P = 0.000). The HIIT resulted in a significant decrease in the gene expression of FIS1 (P = 0.014), DRP1 (P = 0.000), and OPA1 (P = 0.000), along with a non-significant increase in the gene expression of MNF2. Also, HIIT before DOX not only significantly decreased mRNA levels of FIS1 (P = 0.000) and DRP1 (P = 0.000) but also led to a decrease in mRNA levels of OPA1 (P = 0.000) and a non-significant increase in MNF2 mRNA levels. Therefore, preconditioning HIIT could effectively mitigate the changes in the mitochondrial dynamic process caused by DOX (Figure 1).
A, Optic atrophy 1 (OPA1); B, Mfn2; C, FIS1; and D, DRP1 mRNA levels in the left ventricle of rats in the four experimental groups. * P < 0.05; ** P < 0.01: Statistically significant difference from the control group. ## P < 0.01: Statistically significant difference from the doxorubicin (DOX) group
4.2. Proteins Related to Autophagy Process
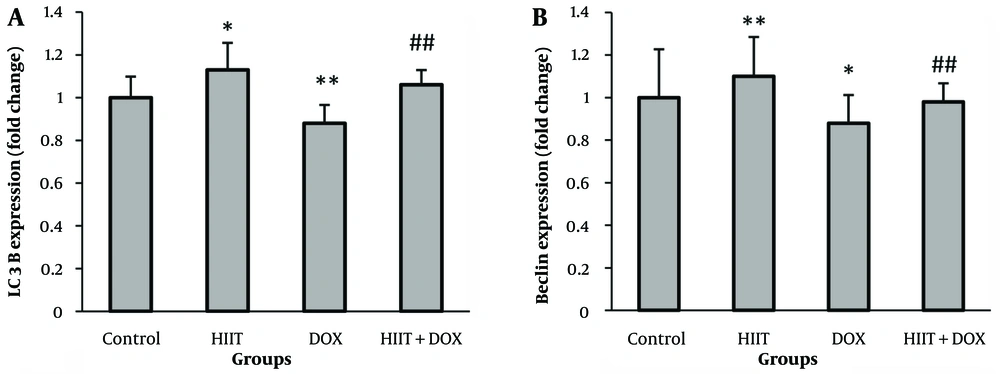
Results showed that LC3II (P = 0.002) and Beclin1 mRNA levels (P = 0.005) decreased significantly after DOX induction. This indicates that DOX led to the inactivation of the autophagy process. Additionally, HIIT resulted in a significant increase in the expression levels of LC3II (P = 0.01) and Beclin1 (P = 0.0001). The HIIT before DOX also significantly increased the LC3II (P = 0.000) and Beclin1 (P = 0.008) mRNA levels. Therefore, HIIT before DOX could effectively modulate DOX-induced alterations in the autophagy process (Figure 2).
5. Discussion
Doxorubicin induction causes cardiotoxicity (14) due to an imbalance in mitochondrial dynamics and the autophagy process, resulting in dysfunctional and fragmented mitochondrial accumulation (2). Studies have shown that DOX increases the levels of DRP1 expression (5, 6), but blocking DRP1 reduces DOX-induced mitochondrial fragmentation and cell death in the heart (6). Furthermore, it has been found that DOX-induced Mfn2 blockade is associated with increased mitochondrial fission and cell death (7). This study observed that DOX resulted in a notable reduction in Mfn2 protein expression levels. Thus, Mfn2 likely plays a key role in controlling mitochondrial dynamic balance. The current study also found that performing HIIT per se and before DOX increased Mfn2 expression and decreased mitochondrial fission-related protein expression.
Contrary to several studies showing that DOX could decrease the expression of fusion-related proteins (MNF1, MNF2, and OPA1) along with mitochondrial fragmentation (5, 22), another study indicates that DOX could increase the expression of OPA1, leading to an increase in defective and fragmented mitochondria (23). In the present study, DOX induction also increased OPA1 expression. Consequently, conflicting results are evident concerning mitochondrial fusion-related proteins, especially in the case of OPA1.
It has been demonstrated that damaged and fragmented mitochondria could be eliminated through a selective process known as autophagy (6, 9). Recent research has indicated that DOX has the potential to enhance autophagy by upregulating the gene expression of autophagy-related proteins (LC3, Beclin1, Pink1, Parkin, and p62) (5). However, conflicting evidence suggests that DOX could hinder the autophagy process in cardiac cells by activating the JNK (24) and Akt/mTOR signaling pathways, reducing levels of Beclin1 (9), and causing subsequent cardiac damage (19). Additionally, this study reveals that DOX could deactivate the autophagy process by decreasing the expression of LC3II and Beclin1.
Research on the effects of DOX on autophagy and mitochondrial dynamics reveals conflicting findings, likely due to two main factors. First, the increase in damaged and fragmented mitochondria induced by DOX could cause an initial rapid growth in the autophagy process within 72 hours (5) by enhancing the expression of key autophagy-related proteins such as Beclin1, LC3II, Pink1, Parkin, p62, S6K1, Atg5, and Atg12 (5, 25). Furthermore, a comprehensive analysis of various research studies shows that while there is an initial increase in the autophagy process, the autophagic flux in cardiomyocytes eventually becomes blocked after several days or weeks due to DOX-induced lysosomal dysfunction (26). This dysfunction is associated with a reduced formation of autophagolysosomes, decreased activity of ULK1/AMP-activated protein kinase (AMPK), and the accumulation of non-degraded autolysosomes (25-27).
The blockage of autophagic flux caused by DOX (27) could cause a modulatory increasing response in mitochondrial fusion (joining depolarized defective and fragmented mitochondria with healthy ones) by upregulating the expression of OPA1, which in turn increases cardiomyocyte death (28). Conversely, OPA1 knockout reduces fusion and accelerates the removal of dysfunctional mitochondria (29). On the other hand, Mfn2 knockout in cardiomyocytes increases dysfunctional mitochondria and impairs mitochondrial autophagy (29). The current study demonstrates that DOX induction significantly increases the expression of OPA1 while decreasing the expression of Mfn2 and disrupting the autophagy process. In contrast, HIIT, both on its own and before DOX, powerfully counters these effects. Thus, the proper interaction between the mitochondrial dynamic and the autophagy process is essential for preserving mitochondrial balance and, consequently, optimal cardiomyocyte function (30).
While strong evidence supports the effect of MCT against CTD (5, 10, 19), few studies investigate HIIT's cardioprotective role and its mechanisms against CTD. Recent studies indicate HIIT before DOX could mitigate cardiotoxicity by reducing serum markers of heart damage, such as LDH and CK-MB levels (14, 31), while enhancing antioxidant defense mechanisms (15, 16). Recent research has indicated that CTD might be partially due to the malfunction of energy metabolism by reducing ATP reserves due to inhibited peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), CaMK, and AMPK signaling activity in cardiomyocytes (25, 32, 33). However, previous studies have also indicated the positive impact of HIIT before DOX on the expression profiles of these genes (32, 34). Furthermore, it has been noted that the pathological elevated expression levels of miR-499, which negatively regulates AMPK and PGC-1α, following DOX treatment, could be reduced by HIIT before DOX in cardiomyocytes (35).
In conclusion, antioxidant defense mechanisms, energy metabolism signaling pathways, the autophagy process, and mitochondrial dynamics play critical roles in the protective effects of HIIT against CTD, which needs further research.
5.1. Conclusions
Research results indicated that eight weeks of HIIT before DOX injection could attenuate DOX-induced disturbances in the mitochondrial dynamics (fission and fusion) and autophagy process. Therefore, HIIT as a non-pharmacological strategy could effectively protect cardiomyocytes against CTD.