1. Background
Sleep is a vital and cyclical physiological process essential for maintaining homeostasis, promoting physical and mental recovery, and optimizing the functioning of the immune, endocrine, and cardiovascular systems (1). In healthy adults, sleep is structured into four to six cycles, each lasting approximately 90 to 100 minutes, alternating between slow-wave sleep non-rapid eye movement (REM) and REM sleep, for an optimal total duration of 7 to 8 hours per night. This rhythm is mainly regulated by two complementary mechanisms: The circadian clock, located in the suprachiasmatic nucleus of the hypothalamus, and the homeostatic pressure of sleep (2). Together, they ensure the periodicity, continuity, and depth of sleep. However, in critically ill patients admitted to intensive care units (ICUs), this equilibrium is often severely disrupted. Numerous studies have highlighted profound alterations in sleep architecture in this population, such as increased sleep fragmentation, frequent awakenings, and lighter sleep stages (3, 4).
Sleep architecture in ICUs is significantly impaired, characterized by significant fragmentation, reduced total duration, decreased or even absent REM sleep, and loss of circadian rhythmicity (5, 6). Several studies have reported that approximately 38.5% to 66% of patients who have been in an ICU experience poor sleep quality, characterized by frequent awakenings and difficulty falling asleep, which can persist for up to a year after hospitalization (7, 8). These alterations are multifactorial: They result not only from the pathophysiological impact of acute illness (systemic inflammation, hypoxia, pain, hemodynamic instability) but also from iatrogenic factors (sedation, analgesia, invasive ventilation, night-time care), psychological factors (stress, isolation, anxiety, absence of loved ones), and environmental factors (noise, artificial light, constant alarms) (9-11). Such disturbances induce a catabolic state, compromise respiratory and immune functions, and contribute to the development of delirium, reflecting a bidirectional relationship between sleep deprivation and cognitive impairment (12-14). Persistent neurocognitive dysfunction has also been reported following ICU stays, highlighting the potentially central role of sleep quality in the patient's overall recovery (15, 16). Frequent sleep disturbances in this context have been linked to higher mortality and poorer clinical outcomes (13, 17).
From a methodological perspective, assessing sleep in ICUs remains particularly challenging. Although polysomnography (PSG) is the gold standard, its implementation in a critical environment is limited due to its technical nature, high cost, and the need for patient cooperation (18). Actigraphy, a less invasive and validated method, provides a partial objective assessment but is still constrained in intensive care environments (19). In practice, sleep self-assessment questionnaires, such as the Richards-Campbell Sleep Questionnaire (RCSQ), are widely used as reliable, simple, and cost-effective tools in clinical ICU settings (20). This questionnaire assesses five subjective dimensions of sleep (latency, depth, efficiency, frequency of awakenings, and overall quality) using a Visual Analogue Scale (VAS). The validated Moroccan Arabic version of the validated Richards-Campbell Sleep Questionnaire (AM-RCSQ) has shown excellent reliability in critically ill patients, especially for longitudinal follow-up and assessing the impact of interventions (21).
2. Objectives
The aim of this study is, therefore, to subjectively assess the quality of sleep in ICU patients using the RCSQ and to identify the sociodemographic, clinical, therapeutic, and environmental factors that are significantly associated with impaired sleep. This study builds upon a previous analysis conducted on the same ICU cohort, which used the Freedman Sleep Questionnaire (FSQ) to explore the impact of environmental factors on sleep quality (22). In contrast, the present work focuses on sociodemographic, clinical, and therapeutic determinants using a different validated instrument, the RCSQ. This complementary approach enables a more comprehensive and detailed understanding of sleep disturbances in critically ill patients.
3. Methods
3.1. Study Design
A cross-sectional observational study was conducted. Data were collected prospectively from patients admitted to the ICUs of three hospitals in the Souss-Massa region of southern Morocco from February 16, 2023, to March 15, 2025.
3.2. Patients
The study included 245 ICU patients.
3.2.1. Inclusion Criteria
Participants eligible for inclusion were adult patients aged 18 years or older, fluent in Arabic, and capable of understanding the study procedures, regardless of the reason for their ICU admission or the severity of their condition. All participants provided informed consent prior to enrollment.
3.2.2. Exclusion Criteria
Patients were excluded from the study if they presented with any of the following conditions: Hearing or speech impairments that could interfere with effective communication; a previously diagnosed dementia or cognitive impairment affecting comprehension; a documented history of substance use disorder, which may influence sleep patterns; or a Glasgow Coma Scale (GCS) score of less than 15, indicating an altered level of consciousness incompatible with reliable self-reporting.
3.3. Sleep Assessment
Sleep quality was assessed using the AM-RCSQ, adapted by Lkoul et al. The tool showed high reliability (Cronbach’s alpha: 0.894 - 0.983) and good validity, with item correlations exceeding 0.4 (21). This 5-item questionnaire evaluates subjective sleep dimensions, including perceived sleep depth, sleep latency, number of awakenings, sleep efficiency, and time awake. Each item is scored on a VAS (0 - 100 mm), with higher scores reflecting better sleep quality. The average score across these five items represents the overall AM-RCSQ score. An additional item on perceived noise disturbance was also included, in line with standard RCSQ practice.
3.3.1. Definition of Sleep Disorders
A total AM-RCSQ score greater than or equal to 50 was considered indicative of good sleep (reliability over 0.92), while a score below 50 denoted poor sleep quality (23).
3.4. Ethical Considerations
This study was approved by the Biomedical Research Ethics Committee (CERB) of the Faculty of Medicine and Pharmacy, Mohammed V University in Rabat (N/R: File No. 154/24), and authorization to collect data was issued by the regional Department of Health and Social Protection of the Souss-Massa region. All participants were informed about the study’s objectives and procedures and provided written informed consent prior to enrollment.
3.5. Data Analysis
A descriptive analysis was performed to characterize the sociodemographic, clinical, and admission variables related to patients' sleep. Categorical variables were presented as frequencies and percentages, while quantitative variables were summarized using means and standard deviations. Comparisons between groups of patients with good and poor sleep quality, defined according to an AM-RCSQ score threshold of 50, were performed using the chi-square test for categorical variables or Fisher's exact test for small sample sizes. Secondly, a multiple regression analysis was conducted to identify factors independently associated with the AM-RCSQ score. The AM-RCSQ score was used as the dependent variable. The explanatory variables included in the model were selected based on their clinical relevance and statistical significance in the univariate analyses. The importance of the multiple regression model was assessed using analysis of variance (ANOVA). The coefficient of determination R² was calculated to quantify the proportion of variance in the AM-RCSQ score explained by the model. The absence of autocorrelation of the residuals was verified using the Durbin-Watson test. The contribution of the explanatory variables to the significant explanation of the AM-RCSQ score was tested using Student's t-test. The absence of multicollinearity was verified by calculating the tolerance and variance inflation factor (VIF) values for each explanatory variable. A tolerance threshold of < 0.3 and VIF > 4 was used to identify multicollinearity issues. A significance threshold of P < 0.05 was used for all statistical analyses, with a 95% confidence interval (95% CI).
4. Results
4.1. Sleep Quality and Sociodemographic Characteristics of Patients
Table 1 shows the distribution of sociodemographic and clinical characteristics of ICU patients stratified by sleep quality, as measured by the AM-RCSQ. The study compares two groups of patients: Those with poor sleep quality (AM-RCSQ < 50) and those with good sleep quality (AM-RCSQ ≥ 50), comprising a total of 245 patients. Of the 245 ICU patients assessed, 183 (74.7%) were classified as having poor sleep quality, while 62 (25.3%) reported good sleep quality. Significant associations were found between sleep quality and age (P = 0.008), gender (P = 0.005), educational level (P = 0.026), physical activity (P = 0.015), and length of ICU stay (P = 0.030). In contrast, no significant associations were observed with BMI categories, marital status, and immunity status.
| Variables | Sleep Quality AM-RCSQ | P b | P c | |
|---|---|---|---|---|
| Poor Sleep Quality; 183 (74.7) | Good Sleep Quality; 62 (25.3) | |||
| Age groups d | 0.008 | - | ||
| Adult | 175 (71.4) | 72 (86.7) | ||
| Elderly person | 70 (28.6) | 11 (13.3) | ||
| Gender | 0.005 | - | ||
| Man | 109 (44.5) | 52 (62.7) | ||
| Woman | 136 (55.5) | 31 (37.3) | ||
| BMI e | - | 0.900 | ||
| Underweight | 45 (18.4) | 19 (22.9) | ||
| Normal | 112 (45.7) | 38 (45.8) | ||
| Overweight | 63 (25.7) | 19 (22.9) | ||
| Obese | 22 (9) | 6 (7.2) | ||
| Morbidly obese | 3 (1.2) | 1 (1.2) | ||
| Marital status | - | 0.172 | ||
| Single | 35 (14.3) | 14 (16.9) | ||
| Married | 180 (73.5) | 66 (79.5) | ||
| Divorced | 10 (4.1) | 1 (1.2) | ||
| Widowed | 20 (8.2) | 2 (2.4) | ||
| Origin | 0.203 | - | ||
| Rural | 123 (50.2) | 49 (59) | ||
| Urban | 122 (49.8) | 34 (41) | ||
| Education level | - | 0.026 | ||
| None | 108 (44.1) | 26 (31.3) | ||
| Primary | 76 (31) | 22 (26.5) | ||
| Secondary | 50 (20.4) | 28 (33.7 | ||
| University | 11 (4.5) | 7 (8.4) | ||
| Physical activity | 0.015 | - | ||
| No | 219 (89.4) | 65 (78.3) | ||
| Yes | 26 (10.6) | 18 (21.7) | ||
| Length of stay (d) | 0.030 | - | ||
| < 5 | 118 (48.2) | 52 (62.7) | ||
| ≥ 5 | 127 (51.8) | 31 (37.3) | ||
Abbreviation: AM-RCSQ, Moroccan Arabic version of the validated Richards-Campbell Sleep Questionnaire.
a Values are expressed as No. (%).
b Exact two-tailed significance of the chi-square statistical test.
c Exact two-tailed significance of Fisher's exact statistical test.
d Age groups: Adult: 18 - 65; older adult: ≥ 65.
e BMI: Underweight: < 18.4; normal: 18.5 - 24.9; overweight: 25 - 29.9; obese: 30 - 34.9; morbidly obese: ≥ 35.
4.2. Sleep Quality and Clinical-Pathological Characteristics of Patients
Table 2 shows that poor sleep quality in ICU patients was significantly associated with clinical factors such as smoking (P = 0.031), use of diuretics (P = 0.045), non-invasive ventilation (NIV) (P = 0.004), oxygenation methods (P = 0.003), and specific pathologies including respiratory failure (P = 0.017), circulatory failure (P = 0.011), diabetes (P = 0.002), and the presence of invasive devices (P < 0.001). Other variables, including reason for admission, type of ICU, use of inotropes or beta-blockers, and kidney failure, were not significantly associated with sleep quality.
| Variables | Sleep Quality AM-RCSQ | P b | P c | |
|---|---|---|---|---|
| Poor Sleep Quality; 183 (74.7) | Good Sleep Quality; 62 (25.3) | |||
| Reason for admission | - | 0.694 | ||
| Respiratory failure | 65 (26.5) | 17 (20.5) | ||
| Circulatory failure | 108 (44.1) | 42 (50.6) | ||
| Metabolic disorders | 59 (24.1) | 20 (24.1) | ||
| Others | 13 (5.3) | 4 (4.8) | ||
| Type of ICU | 0.335 | - | ||
| Medical ICU | 180 (73.5) | 54 (65.1) | ||
| Surgical ICU | 26 (10.6) | 12 (14.5) | ||
| Postoperative ICU | 39 (15.9) | 17 (20.5) | ||
| Smoking | 0.031 | - | ||
| No | 194 (79.2) | 75 (90.4) | ||
| Yes | 51 (20.8) | 8 (9.6) | ||
| Diuretics | 0.045 | - | ||
| No | 228 (93.1) | 71 (85.5) | ||
| Yes | 17 (6.9) | 12 (14.5) | ||
| Inotropes | 0.270 | - | ||
| No | 209 (85.3) | 75 (90.4) | ||
| Yes | 36 (14.7) | 8 (9.6) | ||
| Beta-blockers | 0.404 | - | ||
| No | 219 (89.4) | 77 (92.8) | ||
| Yes | 26 (10.6) | 6 (7.2) | ||
| Corticosteroids | 0.111 | - | ||
| No | 196 (80) | 72 (86.7) | ||
| Yes | 49 (20) | 11 (13.3) | ||
| Covid-19 vaccination | 0.216 | - | ||
| No | 20 (8.2) | 3 (3.6) | ||
| Yes | 225 (91.8) | 80 (96.4) | ||
| Non-invasive ventilation | 0.004 | - | ||
| No | 200 (81.6) | 79 (95.2) | ||
| Yes | 45 (18.4) | 4 (4.8) | ||
| Oxygenation methods | 0.003 | - | ||
| Low flow O2 | 46 (18.8) | 11 (13.3) | ||
| High flow O2 | 73 (29.8) | 12 (14.5) | ||
| Ambient air | 126 (51.4) | 60 (72.3) | ||
| Respiratory disease | 0.017 | - | ||
| No | 177 (72.2) | 71 (85.5) | ||
| Yes | 68 (27.8) | 12 (14.5) | ||
| Heart disease | 0.011 | - | ||
| No | 168 (68.6) | 69 (83.1) | ||
| Yes | 77 (31.4) | 14 (16.9) | ||
| Kidney failure | - | 0.578 | ||
| No | 230 (93.9) | 80 (96.4) | ||
| Yes | 15 (6.1) | 3 (3.6) | ||
| Diabetes | 0.002 | - | ||
| No | 202 (82.4) | 80 (96.4) | ||
| Yes | 43 (17.6) | 3 (3.6) | ||
| Type of diabetes | - | 0.004 | ||
| Type I diabetes | 12 (4.9) | 1 (1.2) | ||
| Type II diabetes | 31 (12.7) | 2 (2.4) | ||
| Non-diabetic | 202 (82.4) | 80 (96.4) | ||
| Pregnancy | < 0.001 | - | ||
| No | 225 (91.8) | 61 (73.5) | ||
| Yes | 20 (8.2) | 22 (26.5) | ||
| Invasive devices | < 0.001 | - | ||
| No | 11 (4.5) | 56 (67.5) | ||
| Yes | 234 (95.5) | 27 (32.5) | ||
Abbreviations: AM-RCSQ, Moroccan Arabic version of the validated Richards-Campbell Sleep Questionnaire; ICU, intensive care unit.
a Values are expressed as No. (%).
b Exact two-tailed significance of the chi-square statistical test.
c Exact two-tailed significance of Fisher's exact statistical test.
4.3. Factors Associated with Sleep Quality
Table 3 summarizes the multiple regression analysis of factors predicting variations in AM-RCSQ scores. Variables were grouped into key categories: Demographic/medical, socio-economic, lifestyle, medical care, pathologies, clinical scores, and admission context. The medical care category yielded the most significant predictors. Specifically, higher magnesium levels (β = 7.471, P = 0.011) and COVID-19 vaccination (β = 7.580, P = 0.013) were positively associated with sleep quality. Conversely, NIV (β = -5.345, P = 0.027) and the use of invasive devices (β = -18.390, P < 0.001) were associated with poorer sleep.
| Predictors | β (IC 95%) a | P b | Tolerance | VIF |
|---|---|---|---|---|
| (Constante) | 61.131 (47.486 - 74.776) | < 0.001 | - | - |
| BMI | -0.01 (-0.166 - 0.146) | 0.897 | 0.272 | 3.671 |
| IMC | 0.162 (-0.178 - 0.503) | 0.349 | 0.864 | 1.158 |
| Gendre | -2.827 (-6.343 - 0.688) | 0.115 | 0.685 | 1.46 |
| Marital status | -0.285 (-2.863 - 2.293) | 0.828 | 0.706 | 1.417 |
| Origin | -1.753 (-4.922 - 1.417) | 0.277 | 0.844 | 1.184 |
| Education level | -0.475 (-2.332 - 1.382) | 0.615 | 0.711 | 1.406 |
| Physical activity | 3.021 (-2.108 - 8.149) | 0.247 | 0.692 | 1.444 |
| Immune status | -0.275 (-5.516 - 4.966) | 0.918 | 0.31 | 3.226 |
| Reason for admission | -1.983 (-4.099 - 0.133) | 0.066 | 0.689 | 1.451 |
| Type of ICU | -1.041 (-3.234 - 1.151) | 0.351 | 0.746 | 1.34 |
| Length of stay | -0.188 (-0.857 - 0.481) | 0.581 | 0.797 | 1.255 |
| Smoking | 0.584 (-3.635 - 4.803) | 0.786 | 0.805 | 1.242 |
| Diuretics use | 2.938 (-3.448 - 9.323) | 0.366 | 0.644 | 1.554 |
| Oxygenation methods | 0.837 (-1.307 - 2.98) | 0.443 | 0.785 | 1.273 |
| Respiratory disease | -2.037 (-6.134 - 2.06) | 0.329 | 0.683 | 1.463 |
| Heart disease | -5.582 (-9.837 - -1.327) | 0.01 | 0.583 | 1.716 |
| Kidney failure | 0.161 (-7.439 - 7.761) | 0.967 | 0.706 | 1.416 |
| Diabetes | 2.026 (-3.359 - 7.412) | 0.46 | 0.605 | 1.653 |
| Pregnancy | 4.828 (-3.713 - 13.368) | 0.267 | 0.26 | 3.85 |
| Invasive devices | -18.39 (-22.543 - -14.238) | < 0.001 | 0.755 | 1.325 |
| VAS pain-score | -2.266 (-2.775 - -1.757) | < 0.001 | 0.799 | 1.252 |
| Charlson-score | 0.063 (-1.607 - 1.733) | 0.941 | 0.428 | 2.334 |
| SOFA-score | -0.024 (-2.271 - 2.223) | 0.983 | 0.644 | 1.552 |
| APACHE II-score | 0.065 (-0.303 - 0.433) | 0.728 | 0.468 | 2.137 |
Abbreviation: VIF, Variance Inflation Factor; VAS, Visual Analogue Scale.
a Unstandardized coefficient with its 95% confidence interval.
b Statistical significance of the student’s t-test.
Among pathologies, heart disease showed a significant negative association (β = -5.582, P = 0.010). A higher VAS pain score also predicted poorer sleep (β = -2.266, P < 0.001). Other categories did not show individually significant associations (p ≥ 0.05). Multicollinearity diagnostics confirmed the robustness of the model, with all tolerance values above 0.260 and VIFs below 4. The highest VIF was noted for pregnancy (3.850), and the lowest tolerance for immune status (0.260), yet neither exceeded critical thresholds, indicating that multicollinearity was not a concern.
4.4. Sleep Quality and the Patient’s Disease
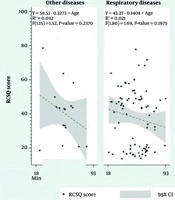
Figure 1 illustrates the linear regression of AM-RCSQ scores as a function of age across different pathological groups. In patients with circulatory disease, age emerged as a significant predictor of sleep quality (R2 = 0.144, P < 0.0001), indicating a moderate negative association. However, for respiratory (R2 = 0.021, P = 0.1975), metabolic (R2 = 0.001, P = 0.7863), and other pathologies (R2 = 0.092, P = 0.237), age was not significantly associated with AM-RCSQ scores, suggesting a weak or negligible explanatory power in these groups.
5. Discussion
Among the 245 participants, 74.7% reported poor sleep quality as measured by the AM-RCSQ, highlighting the high prevalence of sleep disturbances in critical care settings. These findings are consistent with the meta-analysis by Shih et al., which reported similar global prevalence rates. Across the studies reviewed, prevalence ranged from 30% to 88.7%, with the highest values observed in medical ICUs where patients often suffer from more complex or chronic conditions. Post-surgical units, particularly cardiothoracic ICUs, also reported high rates, typically between 46% and 72% (24). The age effect was more pronounced in women, corroborating the work of Al Mutair et al. and Medrzycka-Dabrowska et al., who suggest that older women are more sensitive to insomnia and circadian disorganization, particularly in postmenopausal women (25, 26).
Female gender was associated with poorer sleep quality, which aligns with the results of Wesselius et al., who attributed this difference to hormonal, emotional, and social factors (27). Women hospitalized in the ICU tend to experience heightened anxiety, hypervigilance, and perception of a disruptive environment. However, some studies, such as Vitkova et al., suggest that these differences may be attenuated in critically ill patients, where the severity of comorbidities may overshadow the gender effect (28).
Smoking confirms the deleterious effect of nicotine on sleep quality. Deleanu et al. described nicotine’s negative effects on sleep fragmentation, nocturnal awakenings, and increased risks of obstructive sleep apnea (29). Grigoriou et al. also observed reduced REM sleep in hospitalized smokers, potentially impairing recovery (30).
In terms of pathology, patients with respiratory and circulatory failure, along with diabetes-related diseases, had significantly poorer sleep quality. Stewart et al. explained that respiratory diseases lead to sleep fragmentation due to episodes of dyspnea, hypoxia, and nocturnal desaturation (31). Cardiac conditions such as heart failure or circulatory failure can induce awakenings related to orthopnea or chest pain (32). As for diabetes, its complications, such as polyuria, peripheral neuropathy, or nocturnal hypoglycemia, may contribute to sleep disturbances (33).
Simultaneously, the use of NIV was also associated with poorer sleep quality, consistent with the findings of Le Dinh et al., who reported frequent sleep interruptions due to alarms, noise, and discomfort from the NIV mask (34). The prolonged use of this type of device is also correlated with increased stress and anxiety. Several studies have reported high clinical scores in patients with altered sleep. In the present study, the VAS pain, Charlson, SOFA, and APACHE II scores were all significantly higher, indicating greater pain, comorbidity, and disease severity. Bernat Adell et al. reported a strong correlation between poorly controlled pain and insomnia in ICU patients, advocating for multimodal analgesia (35). Similarly, Naik et al. showed that an APACHE II score > 10 doubles the risk of severe sleep disorders (23).
In summary, this study highlights a high prevalence of sleep disturbance in ICU settings and identifies modifiable contributors, such as pain, invasive devices, and magnesium deficiency, making them priority targets for interventions. These results support the implementation of sleep-conscious care protocols and caregiver training to enhance recovery and reduce hospitalization duration.
5.1. Conclusions
This study underscores the high prevalence of sleep disorders in ICU patients and identifies key intrinsic factors (age, gender, comorbidities, pain, invasive devices) and extrinsic factors (environmental disturbances such as noise and nocturnal interventions). Mitigating these factors by reducing ambient noise and limiting non-urgent nocturnal interventions offers an accessible and practical approach to improving patient sleep. Sleep quality should be recognized as a core component of critical care with direct implications for physiological recovery, length of stay, and overall morbidity. ICU teams must therefore acknowledge these determinants and implement targeted environmental and clinical strategies to optimize patient comfort and outcomes. Further interventional studies are needed to validate the effectiveness of these measures in various critical care settings.
5.2. Limitations
This study presents several limitations. Sleep quality was assessed using subjective, self-reported measures, which may lack precision and are susceptible to recall bias — though this effect is likely minimal due to the short ICU stay. Additionally, the sampling method may limit the generalizability of the findings, and important psychological variables such as stress, anxiety, and depression were not evaluated.
5.3. Strengths
Despite these limitations, the study has notable strengths. It involved a relatively large and diverse ICU patient population, enhancing the internal validity of the findings. The use of a culturally adapted and psychometrically validated sleep assessment tool (AM-RCSQ) ensures methodological reliability. Moreover, the comprehensive analysis of a wide range of clinical, demographic, and treatment-related variables offers valuable insights for ICU practice and future research.