1. Background
Metabolic syndrome (MetS), characterized by disrupted energy metabolism, obesity, insulin resistance (IR), and hypertension, increases the risk of diabetes and neurodegenerative diseases. Lifestyle factors such as poor diet, inactivity, and sleep issues exacerbate these effects (1). In diabetes, disrupted brain cholesterol metabolism promotes amyloidogenic processing of amyloid precursor protein [APP is a membrane protein that is the precursor of the peptide amyloid beta (Aβ), a key component of amyloid plaques found in neurodegenerative diseases], increasing Aβ (a peptide derived from the APP, a transmembrane protein that plays a role in various neuronal processes) levels through enhanced β- and γ-secretase activity in cholesterol-rich lipid rafts. Since the brain contains approximately 25% of the body’s cholesterol, maintaining cholesterol regulation is vital for neuronal function (2). Brain cholesterol metabolism, regulated by the blood-brain barrier, impacts Aβ accumulation and Alzheimer’s disease. Apolipoprotein E (ApoE) facilitates cholesterol transport to 24-hydroxycholesterol (24-OHC) in neurons, and cholesterol-lowering treatments may reduce Alzheimer’s risk (3). The CYP46A1 gene encodes cholesterol 24-hydroxylase, converting cholesterol to 24-OHC for clearance across the blood-brain barrier. Astrocytes supply cholesterol to neurons, and oxysterols regulate cholesterol homeostasis (4, 5). ATP-binding cassette transporter A1 (ABCA1) facilitates cholesterol efflux to apolipoprotein A1 (APOA1) and ApoE, promoting Aβ clearance. Increased ABCA1 reduces Aβ accumulation, while ER stress and hypercholesterolemia exacerbate it. Low-density lipoprotein receptor (LDLR) helps reduce cholesterol, and inhibiting sterol regulatory element-binding protein 2 (SREBP-2) enhances LDLR expression, supporting cholesterol homeostasis (6). Insulin resistance (IR is a pathological condition in which cells in insulin-sensitive tissues in the body fail to respond normally to the hormone insulin or downregulate insulin receptors in response to hyperinsulinemia) and brain cholesterol metabolism are bidirectionally linked. Hypercholesterolemia promotes IR, while Aβ-induced IR disrupts cholesterol synthesis via SREBP. Endoplasmic reticulum stress (ERS is a condition where the ER, the cell’s protein folding and modification factory, becomes overwhelmed and unable to handle the influx of proteins, leading to the accumulation of misfolded or unfolded proteins) activates the unfolded protein response (UPR is activated by the accumulation of unfolded or misfolded proteins in the ER), leading to protein degradation and, if persistent, apoptosis. Cholesterol synthesis in the ER, regulated by 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), is tied to ER stress and Aβ pathology. Sterol buildup triggers HMGR degradation via SREBP2, and elevated activating transcription factor 6 (ATF6) connects ER stress to Aβ processing, with obesity worsening ER dysfunction (7-9).
Physical activity protects against neurodegeneration by reducing ER stress linked to MetS. In mice, exercise activates the UPR without causing cell death, suggesting a protective effect. Exercise regulates ER stress, glucose-regulated protein 78 [immunoglobulin binding protein (BiP), also known as GRP78 or 70 kDa heat shock protein 5 (HSPA5) or (Byun1), is a protein that in humans is encoded by the HSPA5 gene], and apoptotic pathways, improving brain health and reducing neurodegeneration risk in obesity (10). Aerobic exercise enhances cognitive performance by increasing ABCA1 [ATP-binding cassette transporter ABCA1 (member 1 of human transporter sub-family ABCA), also known as the cholesterol efflux regulatory protein (CERP) is a protein which in humans is encoded by the ABCA1 gene] expression, which is vital for brain lipid metabolism and Alzheimer’s disease pathology (11). Long-term exercise improves cognitive function, reduces dementia risk, modulates neuroinflammation, and activates ApoE (a protein involved in the metabolism of fats in the body of mammals. A subtype is implicated in Alzheimer’s disease and cardiovascular diseases) carriers (12). Studies have shown that changes in circadian rhythm can lead to increased cholesterol synthesis and decreased cholesterol uptake, which is associated with increased expression of sterol regulatory element-binding protein 2 [SREBP-2 also known as sterol regulatory element binding transcription factor 2 (SREBF2) is a protein that in humans is encoded by the SREBF2 gene] and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR is the rate-limiting enzyme in the mevalonate pathway, which produces cholesterol and other isoprenoids. It’s the target of statin drugs, which are used to lower cholesterol levels and treat cardiovascular disease). Increased HMGCR and SREBP2 in the brain of MetS mice indicate stimulation of endogenous cholesterol synthesis pathways. Exercise, especially at night, was able to reduce this increase. This improvement may be due to feedback regulation of cholesterol synthesis by increased peripheral cholesterol uptake [increased ABCA1 and low-density lipoprotein receptor (LDL-R) is a mosaic protein of 839 amino acids (after removal of 21-amino acid signal peptide) that mediates the endocytosis of cholesterol-rich low-density lipoprotein (LDL)] (12). Exercise enhances lipid metabolism by lowering soluble Aβ levels and increasing lipoprotein receptors. Moderate-intensity exercise is more effective than low-intensity in improving lipid metabolism and reducing blood lipid levels (13). In diabetes, exercise may reduce Aβ levels, which are associated with Alzheimer’s disease, by regulating insulin signaling and related processes (14, 15). Exercise timing, particularly evening exercise, impacts metabolism by promoting free fatty acid oxidation, reducing glucose production, and enhancing lipolysis (16, 17). The circadian system, driven by the central suprachiasmatic nucleus (SCN) clock and peripheral clocks, links exercise, feeding, and genes (like BMAL1) to metabolism and brain health. Evening exercise enhances fatty acid oxidation, reduces glucose production, and boosts lipolysis. While exercise impacts metabolism, the molecular pathways connecting exercise to brain cholesterol metabolism and Aβ clearance in the hippocampus remain unclear, despite the known relationship between cholesterol metabolism and Aβ regulation (18).
2. Objectives
The present study explored how continuous exercise and its timing (morning vs. evening) affect brain cholesterol metabolism, ER stress, and Aβ plaque formation in the hippocampus of mice with MetS. Using a high-fat diet (HFD) to induce MetS in male NMRI mice, researchers found that exercise modulated key neurodegenerative markers, with timing influencing the extent of these effects.
3. Methods
3.1. Experimental Animals and Groups
This randomized trial experimental study involved 48 male NMRI mice (6 - 8 weeks old; 20 - 25 g), housed under standardized conditions (12-hour light/dark cycle, 23 ± 1°C), with unrestricted access to food and water. The mice were randomly divided into two groups: A control group (n = 16) receiving a standard rodent diet (10% kcal from fat) and a diabetic group (n = 32) fed a HFD (60% kcal from fat) to induce metabolic dysfunction. All procedures adhered to NIH guidelines for the care and use of laboratory animals (NIH Pub. No. 86-23) and were approved by the Research Ethics Committee of the Sports Science Research Institute (IR.SCU.REC.1403.044). After a 2-week acclimatization, 32 male NMRI mice were fed a HFD (60% kcal from fat) for 12 weeks to induce obesity and MetS, while 16 mice remained on a standard diet as controls (19). The MetS was defined by obesity, hyperglycemia, and dyslipidemia. Mice with fasting glucose > 115 mg/dL, HDL < 40 mg/dL, and triglycerides (TG) > 110 mg/dL were classified with MetS (20). Obesity was confirmed using the Lee Index (body weight1/3/body length) × 1000 (19). Mice were then stratified into six groups (n = 8 per group) based on metabolic condition and circadian phase: Control, diabetic, and diabetic + exercise, further divided into early light-phase and early dark-phase subgroups.
3.2. Exercise Protocol
An 8-week low to moderate intensity continuous treadmill training (LMICT) protocol was preceded by a 7-day familiarization phase, where mice were acclimatized to treadmill conditions (10 - 25 min/day at 5 - 10 m/min) to minimize stress. Non-exercise controls were similarly placed on the treadmill without running. Exercise sessions were conducted 5 days per week, either in the morning (9 - 10 AM) or evening (7 - 8 PM). Maximum running speed (Vmax) was assessed before the intervention, at week 6, and at week 8 to adjust training intensity and ensure progressive overload (21). Training intensity was set at 50 - 60% of Vmax, with session durations of 30 - 60 minutes, including warm-up and cool-down periods (Table 1). Mice completed an 8-week treadmill training protocol, starting at 13 m/min for 30 minutes. Speed increased by 1 m/min weekly, and duration by 10 minutes biweekly, reaching 60 minutes at 20 m/min. Training occurred 5 days per week at 50 - 60% of maximal aerobic capacity (Vmax) (Table 1). To control for environmental and handling stress, non-exercising mice were placed on a stationary treadmill in the same room as the exercise groups and remained inactive for 60 minutes during each scheduled session in both light and dark phases (Figure 1). Forty-eight hours after the final exercise session and following 8 hours of fasting, mice were anesthetized with xylazine (10 mg/kg) and ketamine (90 mg/kg). Cardiac blood was collected, clotted, and centrifuged at 5000 rpm for 10 minutes; serum was stored at -20°C. Brains were then extracted, and hippocampi were dissected under sterile conditions, snap-frozen in liquid nitrogen, and stored at -80°C.
3.3. Serum Biochemical Analysis
Fasting blood glucose and lipid profile (TG, total cholesterol, HDL, LDL) were measured by enzymatic colorimetric assays (Biorex Fars, Iran). Serum insulin was quantified using an enzyme-linked immunosorbent assay (ELISA) kit (Sunlong Biotech Co., China). Insulin resistance was calculated via the homeostasis model assessment (HOMA-IR) using the formula: HOMA-IR = [fasting glucose (mmol/L) × fasting insulin (µU/mL)]/22.5. Tissue samples (20 mg) were homogenized in 200 µL of lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% Triton X-100, 1 mM NaF) with protease inhibitors, using a homogenizer (Heidolph) at 2500 rpm for 1 minute on ice. The homogenate was centrifuged at 10,000 rpm for 15 minutes at 4°C, and the supernatant was stored at -80°C. Protein concentration was measured using the Bradford assay.
3.4. Western Blot Analysis
Protein expression levels of CYP46a1 (cholesterol 24-hydroxylase, also commonly known as cholesterol 24S-hydroxylase, cholesterol 24-monooxygenase, CYP46, or CYP46A1, is an enzyme that catalyzes the conversion of cholesterol to 24S-hydroxycholesterol), ABCA1, HMG-CoA reductase, LDLR, SREBP-2, and Aβ-42 were assessed by Western blotting. Tissue lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors, centrifuged (10,000 rpm, 15 min, 4°C), and protein concentrations were measured using the Bradford assay. Equal protein amounts (20 µg) were resolved via 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking, membranes were incubated overnight at 4°C with specific primary antibodies (Table 2), followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (mouse anti-rabbit IgG-HRP: sc-2357, Santa Cruz) diluted 1:500 in blocking solution for 2 hours. Detection was performed using enhanced chemiluminescence (ECL), and band intensities were quantified with Fusion X software, normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and expressed as fold change versus control.
| Target | Company | Catalog Number |
|---|---|---|
| HMGCR | Novus Biological | NB-100125 |
| Aβ-42 | Cell signaling | Sc-271583 |
| CYP46A1 | Santa Cruz Biotechnology | Sc-515647 |
| ABCA1 | Santa Cruz Biotechnology | Sc-74465 |
| SREBP2 | Cell signaling | 1F-8 |
| LDLR | Santa Cruz Biotechnology | Sc-376938 |
| GAPDH | Cell signaling | #5174 |
Antibodies Used for Western Blot Analysis
3.5. Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis of Endoplasmic Reticulum Stress-Associated Genes
Expression levels of activating transcription factor 6 (ATF6 is a protein that, in humans, is encoded by the ATF6 gene and is involved in the unfolded protein response), GRP78, and PKR-like ER Kinase (PERK is a kinase located in the ER that plays a crucial role in the unfolded protein response) were analyzed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Total miRNA was isolated using the Minute™ miRNA isolation kit (TIANGEN, China), and RNA purity was confirmed by 260/280 nm absorbance (≥ 1.8). Complementary DNA (cDNA) synthesis was performed using the miRcute™ first-strand cDNA synthesis kit via polyadenylation-based reverse transcription. The qRT-PCR was conducted with SYBR® Green master mix (ParsTous, Iran) on a StepOnePlus™ system (Applied Biosystems, USA), using primers from sRNAPrimerDB. The cycling protocol included initial denaturation at 95°C for 5 minutes, followed by 45 cycles of 94°C for 15 seconds and 60°C for 25 seconds. All reactions were run in triplicate with negative controls. Relative expression was calculated using the 2−ΔΔCt method, normalized to U6 snRNA, with confirmed primer efficiencies (22). Sequences of primers used in the current study are shown in Table 3.
| Gene Name | Sequence | Size (bp) | Accession No. |
|---|---|---|---|
| ATF6 | 138 | NM-001081304 | |
| ATF6-mice-F | GTCCAAAGCGAAGAGCTGTCTG | ||
| ATF6-mice-R | AGAGATGCCTCCTCTGATTGGC | ||
| GAPDH | 150 | NM-008084 | |
| GAPDH-mice-F | CATCACTGCCACCCAGAAGACTG | ||
| GAPDH-mice-R | ATGCCAGTGAGCTTCCCGTTCAG | ||
| GRP78 | 121 | NM-022310 | |
| GRP78-mice-F | TGTCTTCTCAGCATCAAGCAAGG | ||
| GRP78-mice-R | CCAACACTTCCTGGACAGGCTT | ||
| PERK | 115 | NM-010121 | |
| PERK-mice-F | CCGATGTCAGTGACAACAGCTG | ||
| PERK-mice-R | AAGACAACGCCAAAGCCACCAC |
Characteristics of Primers Used in the Present Study
3.6. Methods of Use for Cholesterol and Triglycerides
Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping gene for qPCR normalization. HDL-C and TG levels were measured using ISO 13485:2016-certified assay kits (Pars Peyvand Co., Iran), and total cholesterol was assessed using a point end colorimetric-enzymatic assay (CHOLESTEROL-*SL). Serum HDL-C was also quantified using a photometric assay kit from Pars Azmoun Co.
3.7. Data Analysis Method
Data were analyzed using GraphPad Prism v9 (GraphPad Software, San Diego, CA). Normality and homogeneity of variance were evaluated using the Shapiro-Wilk and Levene’s tests, respectively. Two-way analysis of variance (ANOVA) was employed to assess the effects of exercise timing and diabetic status, followed by Tukey’s post hoc test for multiple comparisons. Pearson’s correlation was used to examine associations between variables. Results are expressed as mean ± SD, with significance thresholds set at P < 0.05, P < 0.01, P < 0.001, and P < 0.0001.
4. Results
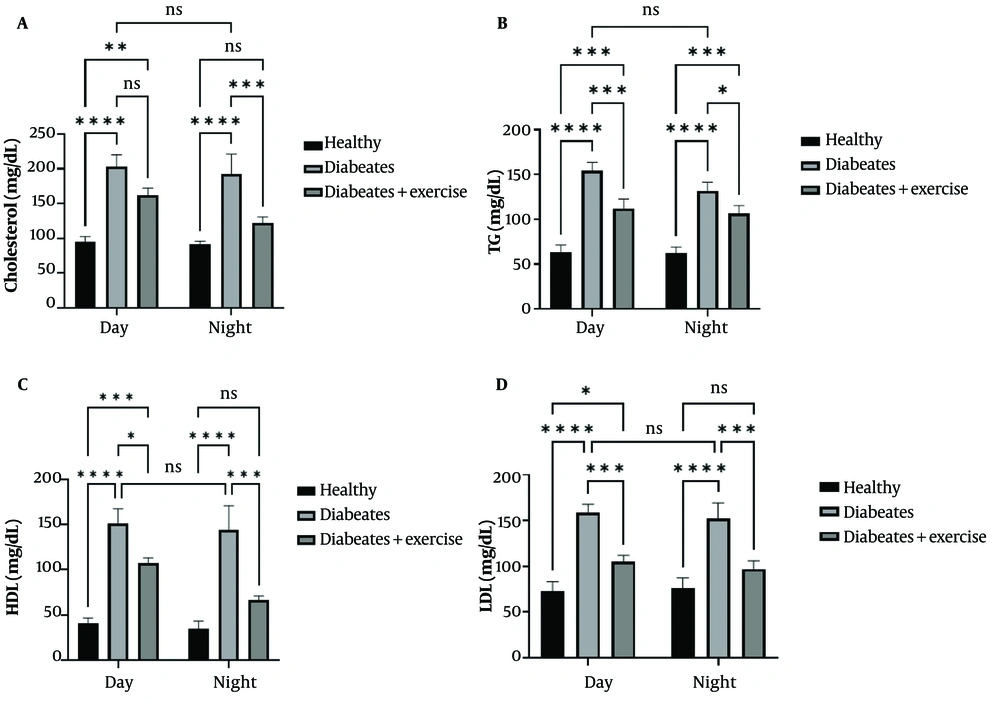
4.1. Lipid Profile Modulation in Diabetic Mice with Exercise
Diabetic mice exhibited significantly higher serum total cholesterol, TG, HDL, and hippocampal LDL levels compared to controls during both light and dark phases (P < 0.0001). Exercise training significantly reduced cholesterol levels during the dark phase (P < 0.001), with no changes during the light phase. Exercise also reduced TG levels in both phases (light: P < 0.001; dark: P < 0.01), HDL levels (light: P < 0.01; dark: P < 0.001), and hippocampal LDL levels (P < 0.001) in diabetic mice. No significant diurnal variations were observed in lipid parameters among exercise-trained diabetic mice (Figure 2).
Effect of continuous training in the morning and evening on cholesterol, triglycerides (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels in different groups during the day and night. Samples were analyzed twice a day and night 48 hours after the final training. In rats with metabolic syndrome (MetS) (results are presented as mean ± standard deviation (number = 2/each group). Statistically significant differences between groups are denoted as follows: * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
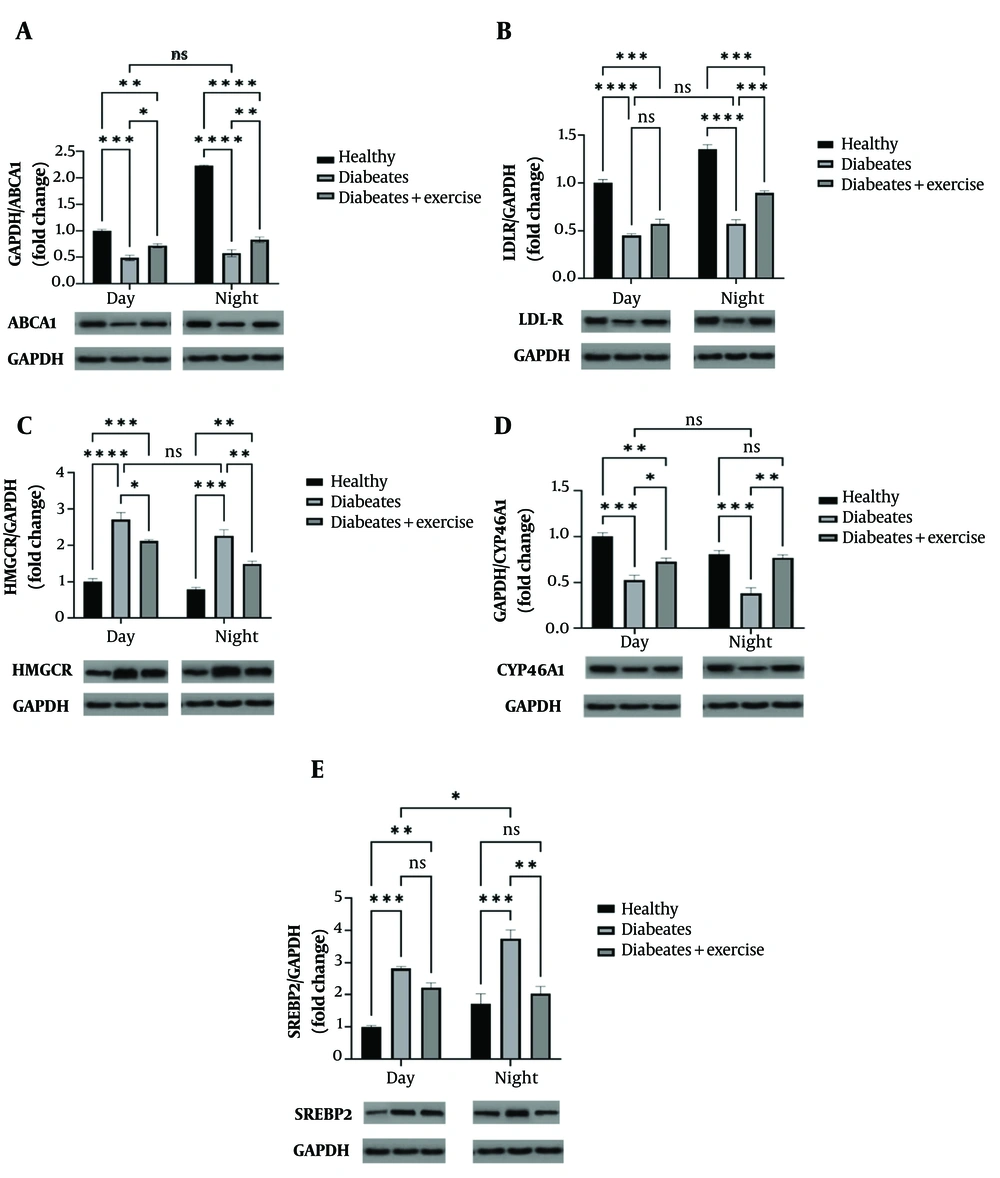
4.2. Exercise Effects on Brain Cholesterol-Related Proteins in Metabolic Syndrome Mice
In MetS mice, protein levels of ABCA1 and LDL-R were significantly decreased during both the light and dark phases (P < 0.001 to P < 0.0001). Exercise training significantly increased ABCA1 expression in both phases (day: P < 0.01; night: P < 0.05), while LDL-R expression was significantly upregulated only at night (P < 0.001). HMG-CoA reductase (HMGCR) levels were significantly elevated in the MetS group (day: P < 0.0001; night: P < 0.001), but exercise suppressed its expression in both phases (day: P < 0.01; night: P < 0.05). CYP46A1 expression, reduced in MetS mice (P < 0.001), was restored by exercise during both phases (day: P < 0.01; night: P < 0.05). SREBP2 levels were elevated in MetS mice (P < 0.001), with exercise further enhancing its expression only at night (P < 0.05). A significant diurnal variation in SREBP2 expression was observed in MetS mice, with higher levels at night (P < 0.01) (Figure 3).
Effect of morning and evening continuous exercise on brain cholesterol-related protein expression (ABCA1, LDLR, HMGCoA, CYP46A1, and SREBP2) in a mouse model of metabolic syndrome (MetS). Protein expression levels were quantified by Western blot analysis in groups subjected to exercise at different times of day. Representative cropped blot images are shown, with GAPDH used as a loading control. Tissue samples were collected 48 hours after the final exercise session, conducted either in the morning or evening. Data are presented as mean ± standard deviation, per group. Statistically significant differences between groups are denoted as follows: * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
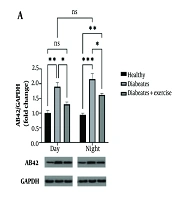
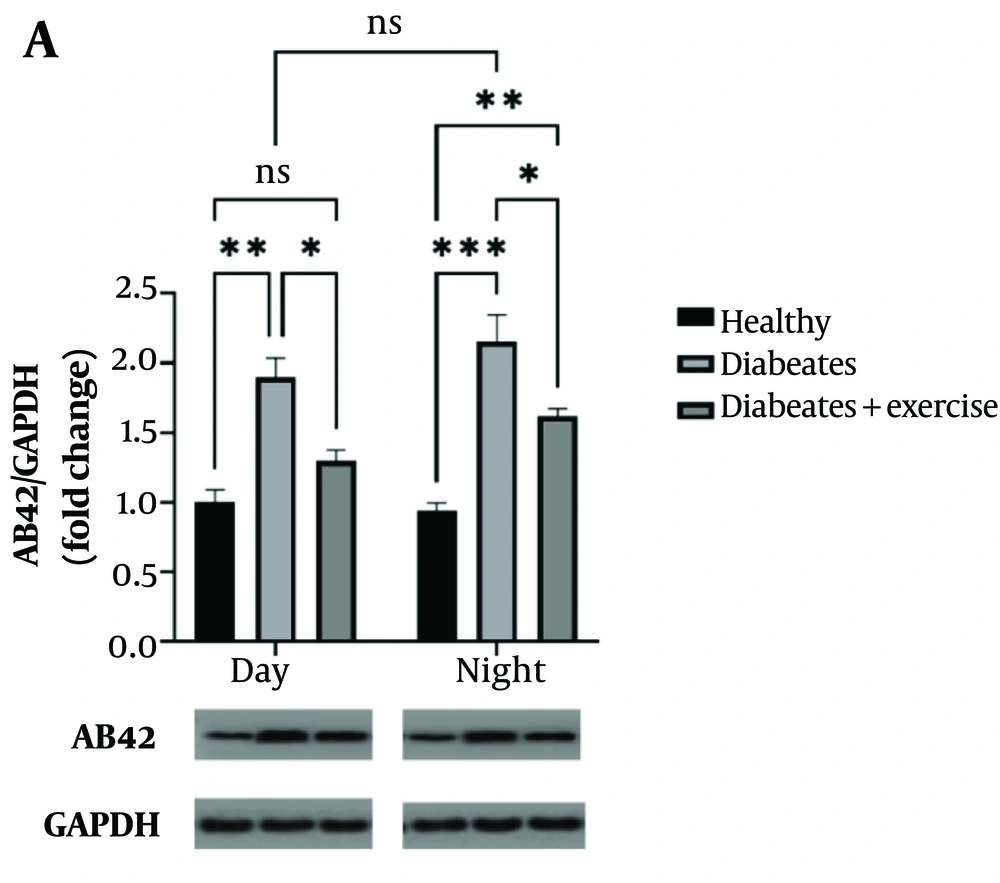
4.3. Chronobiological Effects of Exercise on Amyloid-beta
Amyloid-beta protein levels were significantly elevated in MetS mice during both the day (P < 0.05) and night (P < 0.001) compared to controls. Exercise training reduced Aβ-42 levels at both time points (day: P < 0.01; night: P < 0.01), although the nighttime reduction did not reach statistical significance when compared to untreated MetS mice (Figure 4).
This study examines the effect of continuous exercise in the morning and evening on amyloid-beta (Aβ-42) protein expression in mice with metabolic syndrome (MetS). Amyloid-beta protein levels were assessed using Western blot analysis, with GAPDH serving as a loading control. The samples were collected 48 hours after the final exercise session. The results show significant differences in Aβ-42 expression between the exercise groups, with statistical significance indicated by asterisks corresponding to P-values of * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. These findings highlight the influence of exercise timing on Aβ-42 levels in the context of MetS.
4.4. Chronobiological Effects of Exercise on Endoplasmic Reticulum Stress-Related Genes in Metabolic Syndrome Mice
In MetS mice, expression levels of ATF6, GRP78, and PERK were significantly elevated during both the light and dark phases compared to controls. Exercise training attenuated this upregulation: ATF6 expression was reduced during both phases (day: P < 0.05; night: P < 0.001), GRP78 expression decreased significantly at night (P < 0.0001), and PERK expression was significantly downregulated during both phases (day: P < 0.01; night: P < 0.001). Additionally, a diurnal variation in GRP78 and PERK expression was observed, with higher levels at night (P < 0.001 and P < 0.01, respectively), suggesting a circadian rhythm in ER stress gene expression (Figure 5).
This figure illustrates the effect of continuous morning and evening exercise on the expression of endoplasmic reticulum (ER) stress-related genes [activating transcription factor 6 (ATF6), glucose-regulated protein 78 (GRP78), and protein kinase RNA-like endoplasmic reticulum kinase (PERK)] in mice with metabolic syndrome (MetS). The gene expression levels were measured using quantitative real-time PCR. The results show the mean expression levels with their standard deviation for each experimental group, and statistical significance is denoted by asterisks (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001). Notably, the ATF6 gene expression was assessed in hippocampal tissue at both daytime and nighttime intervals, reflecting the impact of exercise timing on ER stress regulation.
5. Discussion
This study shows that aerobic exercise at different times (day vs. night) improves lipid profiles, regulates hippocampal cholesterol-related proteins, and reduces ER stress in MetS mice. The timing of exercise plays a key role, emphasizing the chronobiological effects on brain cellular mechanisms. In a MetS animal model, increased cholesterol, TG, and low-density lipoprotein (LDL), along with decreased high-density lipoprotein (HDL), indicated significant lipid metabolism disruption (22). Regular exercise, especially at night (aligned with the circadian rhythm of nocturnal animals), effectively lowered TG and LDL levels while enhancing key cholesterol-regulating proteins, including ABCA1 and LDL receptor (LDL-R), in the hippocampus (23). Exercise enhances the expression of genes and proteins involved in cholesterol uptake and reverse transport, such as ABCA1, which facilitates the transfer of cholesterol from nerve cells to HDL. Additionally, increased LDL-R promotes the uptake of LDL cholesterol into the brain, reducing cholesterol accumulation in both brain and peripheral tissues. These findings are consistent with studies by Enkhmaa et al. and Revilla et al. (24, 25)
Endoplasmic reticulum stress, caused by the accumulation of misfolded proteins, activates UPR pathways, which can lead to neuronal apoptosis under chronic conditions (25). In HFD-fed mice, there was a significant increase in the expression of ER stress genes, including ATF6, GRP78, and protein kinase RNA-like endoplasmic reticulum kinase (PERK), indicating UPR activation (26). Regular exercise, especially at night, reduced the expression of these stress-related genes. This effect may result from improved cellular metabolism, decreased protein and lipid load, increased autophagy, enhanced mitochondrial function, and reduced reactive oxygen species (ROS) levels. In our study, diabetic mice that underwent exercise showed decreased expression of ATF6, GRP78, and PERK, supporting these findings (27).
Cholesterol in the brain is synthesized mainly by neurons and astrocytes and plays a critical role in maintaining cell membrane structure and synaptic function (28). Key proteins such as ABCA1 and LDL-R are involved in the regulation of cholesterol homeostasis. Studies have shown that disruption of the circadian rhythm can lead to increased cholesterol synthesis and decreased its uptake, which is associated with increased expression of SREBP2 and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) (29). Increased HMGCR and SREBP2 in the brain of MetS mice indicate stimulation of endogenous cholesterol synthesis pathways. Exercise, especially at night, was able to reduce this increase. This improvement could be due to the feedback regulation of cholesterol synthesis by increased peripheral cholesterol uptake (increased ABCA1 and LDL-R) (30).
Amyloid-beta is a degradation product of APP, which is increased under the influence of oxidative stress and impaired cholesterol synthesis. Improving brain cholesterol metabolism and reducing ER stress can reduce the processes leading to Aβ aggregation. Amyloid-beta protein accumulation is a hallmark of Alzheimer’s disease (31). Studies have shown that impaired cholesterol metabolism can lead to increased Aβ-42 production. Increased Aβ-42 protein in the HFD model is a key indicator of the occurrence of Alzheimer’s-like pathology. The findings showed that exercise significantly reduced this protein, without much difference between day and night exercise (32, 33). Regular exercise can reduce Aβ-42 production by improving cholesterol metabolism and reducing ER stress (27). In our study, a decrease in Aβ-42 levels was also observed in trained diabetic mice, indicating a positive effect of exercise on reducing the accumulation of this protein.
Consistent with prior studies (34, 35), we observed that key cholesterol metabolism proteins, such as ABCA1 and LDL-R, exhibit time-of-day-dependent expression patterns. Notably, exercise-induced upregulation of LDL-R occurred exclusively during the night phase, reflecting circadian control over receptor-mediated cholesterol uptake pathways. This is in agreement with Goodwin et al. (36), who demonstrated nocturnal peaks in LDL-R expression correlating with increased lipid clearance during the active phase in rodents. Similarly, the enhanced nocturnal expression of SREBP2 upon exercise aligns with findings that this master regulator of cholesterol biosynthesis is under circadian modulation (37), possibly to meet increased metabolic demands during the dark phase. Our data showing exercise-mediated reduction of Aβ-42 in both day and night phases corroborate previous evidence supporting physical activity as a neuroprotective intervention mitigating amyloid pathology (38). The somewhat attenuated reduction in Aβ-42 at night suggests potential circadian influences on amyloid clearance mechanisms, an emerging concept supported by recent chronobiology studies (39).
Regarding ER stress markers (ATF6, GRP78, PERK), elevated expression during the night phase and their suppression by exercise align with prior reports that ER stress pathways exhibit circadian oscillations, with higher activity during the active phase (40). The greater nocturnal attenuation of ER stress genes by exercise reinforces the hypothesis that timing of physical activity may optimize cellular stress recovery, consistent with findings by Sato et al. (41) demonstrating time-dependent modulation of UPR pathways by exercise.
Collectively, these results support the growing body of literature emphasizing chronobiology as a critical factor in metabolic and neurodegenerative disease pathophysiology. Moreover, the differential effects of exercise across diurnal phases underscore the potential for chronotherapeutic approaches in managing MetS and its neurological complications by aligning interventions with endogenous circadian rhythms. This study demonstrates that physical activity, particularly during the evening, is more effective than other time periods in improving IR, ER stress, and Aβ-42 accumulation in the hippocampus associated with MetS. One of the limitations of the present study was the lack of dietary intake control. Additionally, other circadian phases such as the afternoon were not examined, nor were other circadian rhythm-related genes assessed. Furthermore, this study did not allow for investigation of circadian rhythms in other organs or the application of different exercise intensities and training models. Therefore, future studies addressing these aspects are recommended.
5.1. Conclusions
This study explores the effect of exercise timing on MetS-induced hippocampal dysfunction. It finds that evening exercise is more effective than other times in improving IR, ER stress, cholesterol regulation, and Aβ-42 accumulation. The results highlight the importance of considering both the type and timing of interventions, supporting the role of circadian medicine in optimizing metabolic and cognitive health.

![This figure illustrates the effect of continuous morning and evening exercise on the expression of endoplasmic reticulum (ER) stress-related genes [activating transcription factor 6 (ATF6), glucose-regulated protein 78 (GRP78), and protein kinase RNA-like endoplasmic reticulum kinase (PERK)] in mice with metabolic syndrome (MetS). The gene expression levels were measured using quantitative real-time PCR. The results show the mean expression levels with their standard deviation for each experimental group, and statistical significance is denoted by asterisks (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001). Notably, the ATF6 gene expression was assessed in hippocampal tissue at both daytime and nighttime intervals, reflecting the impact of exercise timing on ER stress regulation. This figure illustrates the effect of continuous morning and evening exercise on the expression of endoplasmic reticulum (ER) stress-related genes [activating transcription factor 6 (ATF6), glucose-regulated protein 78 (GRP78), and protein kinase RNA-like endoplasmic reticulum kinase (PERK)] in mice with metabolic syndrome (MetS). The gene expression levels were measured using quantitative real-time PCR. The results show the mean expression levels with their standard deviation for each experimental group, and statistical significance is denoted by asterisks (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001). Notably, the ATF6 gene expression was assessed in hippocampal tissue at both daytime and nighttime intervals, reflecting the impact of exercise timing on ER stress regulation.](https://services.brieflands.com/cdn/serve/3170b/cd76ae234cfcb633f6d75c0385e2af8fd2658a45/jjcmb-162429-i005-F5-preview.webp)