1. Background
The Apiaceae family is one of the largest plant families, renowned for its rich aromatic plants. In Iraq, this family comprises approximately 60 genera and more than 150 species (1). Cachrys is a perennial plant belonging to the Apiaceae family, consisting of about 15 known taxa, and is widespread from Central Asia to southern Europe and northern Africa (2-5). According to the Iraqi and Cyprus Flora, Hippomarathrum scabrum (Fenzl) Boiss., Ferulago scabra Fenzl, Bilacunaria scabra (Fenzl), and Ferula scabra are synonyms of Cachrys scabra (Fenzl) Meikle (1, 3, 6). In Iraq, only two species, C. scabra (Fenzl) Meikle and C. papillaris Boiss., have been identified (1). The Cachrys genus consists of perennial herbaceous plants with deeply dissected leaves. Its stems are aromatic, finely and sparingly scabrid, especially around the nodes, well-branched, and robust, reaching approximately 1.5 meters. The lower leaves have broad sheaths, are long-petiolate, triangular-ovate in outline, with numerous thread-like, acute leaflets (1, 7, 8).
There is limited information in the literature regarding the traditional medicinal uses of Cachrys species for treating various diseases. Cachrys libanotis species Linn. has been used as a main component in Algerian traditional herbal medicine to treat rheumatism and gout (9, 10). In the Caucasus and Turkey, the aerial parts of Cachrys species are used to treat digestive disorders, intestinal worms, and for wound healing, as well as a condiment in salads (6, 11). Furthermore, in Serbia and surrounding regions, the roots of the plant are used to treat hemorrhoids (6).
In recent decades, research on Cachrys has primarily focused on its biological activities, including antimicrobial, enzyme inhibitory, antioxidant, anti-inflammatory, cytotoxic, and phototoxic activities, due to the presence of numerous chemical compounds such as terpenes, furanocoumarins, coumarins, and sterols (4, 5, 12-14). For instance, furocoumarins, an active component of Cachrys trifida, can inhibit COX and LOX pathways of arachidonate metabolism, thereby exerting a potent anti-inflammatory effect (13). Additionally, Cachrys essential oil (EO) exhibits various pharmacological effects due to its complex bioactive compounds (5, 7).
2. Objectives
The present study aimed to determine the total phytochemical content of petroleum ether and ethanol extracts of C. scabra, as well as its active EO components, using the GC-MS technique. This analysis aims to further explore the antimicrobial and antioxidant capabilities of C. scabra.
3. Methods
3.1. Plant Materials
Plant materials were collected in June 2022 during the flowering period in Halgurd Mountain, the second-highest mountain in Iraq, located in Erbil province (latitude: 36:42.60247N, longitude: 44:52.19472E, altitude: 2268 m). A voucher specimen has been deposited by assistant professor Dr. Abdullah Shukur Sardar from the College of Education, Salahaddin University-Erbil. After collection, the plant parts were cut into small pieces, dried at room temperature, fragmented, and stored in airtight dark tubes.
3.2. Preparation of Plant Extract
Each plant part (leaf and flower) was ground into a dry powder and extracted using a 100 mL solvent with increasing polarity [petroleum ether (40 - 60%) and ethanol (99.9%)] for three days at room temperature, with the process repeated three times with regular stirring intervals. The solution was vacuum filtered through Whatman No. 1 filter paper, and the solvent was evaporated using a rotary evaporator to yield the crude extract.
3.3. Phytochemical Screening of Cachrys scabra Extracts
3.3.1. Detection of Terpenoids
In a test tube containing 2 mL of chloroform, 5 mL of extract was added, followed by the addition of 2 mL concentrated H2SO4, which forms a layer. A reddish-brown coloration at the interface indicates the presence of terpenoids (15).
3.3.2. Alkaloid Test
A brownish-red precipitate indicating the presence of alkaloids was formed after adding 0.4 mL of HCl (1%) to 10 mL of the extract solution, followed by 6 drops of Dragendorff’s reagent (15).
3.3.3. Phenolics Detection
A small amount of the plant extract solution was mixed with 1 mL of water in a test tube, and 1 to 2 drops of 10% lead acetate were added. A white precipitate indicates a positive test (16).
3.3.4. Flavonoid Detection
Two to five drops of diluted NaOH (10%) were added to a small amount of each plant extract. The immediate development of an intense yellow color indicates the presence of flavonoids (15).
3.3.5. Saponin Detection
In a test tube, 10 mL of the plant extraction solution was added. After two minutes of vigorous manual shaking, the presence of saponin was determined by the formation of stable foam (16).
3.3.6. Tannin Detection
Five mL of the flower and leaf extract was placed in a test tube, and then 2 drops of 1% FeCl3 solution were added. A greenish-black precipitate indicates the presence of tannins (16).
3.4. Isolation of Essential Oils
Essential oils were obtained by steam distillation from dried Cachrys leaves and flowers using a Clevenger apparatus (17). The EOs were stored in airtight tubes, wrapped in aluminum foil, and kept in a freezer at 4°C before use.
3.5. Gas Chromatography-Mass Spectrometry Analysis
An Agilent 6890 Gas Chromatograph coupled to a quadrupole mass spectrometer detector (Hewlett Packard 5973) and a Varian 3400 Gas Chromograph 17A with a flame ionization detector (GC-FID) were employed to perform all chromatographic analyses. The capillary columns were used under the following conditions: (i) DB-5 (50 m × 0.25 mm × 0.25 mm) with an initial oven temperature programmed from 60°C, increasing at 3°C/min-1 until a final temperature of 280°C. Injector and detector temperatures were maintained at 280°C. The nitrogen flow rate was 1.0 mL/min-1, and desorption was performed in split mode (18).
3.5.1. Component Identification
The GC retention indices for the components of the EO were defined to identify the compounds, applying n-alkanes (C6 - C30) as standards (19). The compounds were identified by comparing their retention indices with those previously published in the literature and by comparing their mass spectral data with the Adams Library and published mass spectra (20). To quantify the components of the EO, relative area percentages obtained by GC-FID were used without correction factors. The analysis was repeated three times independently, and the mean values were reported.
3.6. Microbial Strain and Activation Method
Four bacterial strains, including Staphylococcus aureus ATCC 6538P, clinical S. aureus, Pseudomonas aeruginosa ATCC 9027, and clinical P. aeruginosa, were selected for antibacterial screening. Bacterial strains were obtained from the Media Diagnostic Health Center in Erbil. Yeast species, including Candida albicans, C. tropicalis, C. parapsilosis, and C. guillermondii, were obtained from the College of Science, Salahaddin University-Erbil. The yeasts were identified by Dr. Hero Muhammed Ismael. Mueller-Hinton broth (MHB) and sabouraud dextrose broth (SDB) media were used to cultivate bacteria and fungi, respectively. A single colony of each strain was picked and transferred to 1.5 mL of broth medium and incubated in a shaker at 37°C for 24 hours at 100 - 120 rpm. The inoculum of the culture solution was adjusted to the McFarland scale of 0.5 and confirmed by spectrophotometric reading at 580 nm.
3.7. Biological Activity of Cachrys scabra Extracts
3.7.1. Micro-well Dilution Evaluation
The minimum inhibitory concentration (MIC) of C. scabra extracts and EO was determined for all strains as previously described (17), with slight modifications. Suspensions of test strains were prepared from fresh overnight cultures and transferred into normal saline (0.9% NaCl) under aseptic conditions. Twenty μL of strain culture at a density of 1.5 × 106 CFU/mL was added to the wells. Ninety-six well-cultured plates were used for the assay, which was carried out in 96-well sterile microplates. Serial two-fold dilutions of extracts and EO were prepared. The volume of dispensing extract was 100 μL per well, along with 100 μL of MHB and SDA for bacterial and fungal suspensions, respectively. The absorbance for each well was measured at 630 nm before incubation using an enzyme-linked immunosorbent assay (ELISA) Reader (BioTech, Vermont, USA). The plates were then incubated under shaking conditions (100 - 120 rpm) at 37°C for 24 hours. The absorbance was re-measured after incubation to compare with the initial measurement. The MIC was calculated at the breakpoint concentrations by comparing the absorbance before and after incubation. Dimethyl sulfoxide (DMSO) and broth media were used as negative controls. The tests were performed in triplicate.
3.7.2. 1,1-diphenyl-2-picrylhydrazyl Scavenging Activity
The antioxidant activity of the sample was estimated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical test according to the method described by (9). One hundred μL of each sample at various concentrations and the positive control, ascorbic acid, were added to 100 μL of the DPPH methanolic solution (0.04%), except for the blank well. The mixtures were vortexed, incubated at room temperature for 30 minutes in the dark, and their absorbance was measured at 517 nm. The percentage of inhibition was calculated against the blank using the following equation:
Where (As) is the absorbance value of the sample and (Ac) is the absorbance of the control reaction. The IC50 value (mg/mL) was defined as the concentration required to scavenge 50% of free radicals present in the test solution.
3.8. Statistical Analysis
Results are expressed as mean values and analyzed using one-way analysis of variance (ANOVA). The antioxidant concentration providing 50% inhibition (IC50) was calculated by non-linear regression using GraphPad Prism Software Version 7 (San Diego, CA, USA). A P-value of < 0.05 was considered statistically significant.
4. Results and Discussion
4.1. Phytochemical Constituents of Cachrys scabra
The phytochemical qualitative test of C. scabra leaf and flower extracts showed that the components varied depending on the type of solvent used for extraction, as shown in Table 1. The results revealed that both petroleum ether and ethanol extracts are similar in terms of terpenes, alkaloids, phenolics, and flavonoids. However, saponins and tannins were not detected in our study. The results are consistent with the literature, indicating the presence of several secondary metabolites known to possess antifungal, antibacterial, and therapeutic properties (4, 5, 8, 21).
| Extraction Type and Plant Part | Terpenes | Alkaloids | Phenols | Flavonoids | Saponins | Tannins |
|---|---|---|---|---|---|---|
| Petroleum Ether | ||||||
| Leaves | + | + | + | + | - | - |
| Flowers | + | + | + | - | - | - |
| Ethanol | ||||||
| Leaves | + | + | + | + | - | - |
| Flowers | + | + | + | + | - | - |
4.2. Chemical Composition of Cachrys scabra Essential Oil
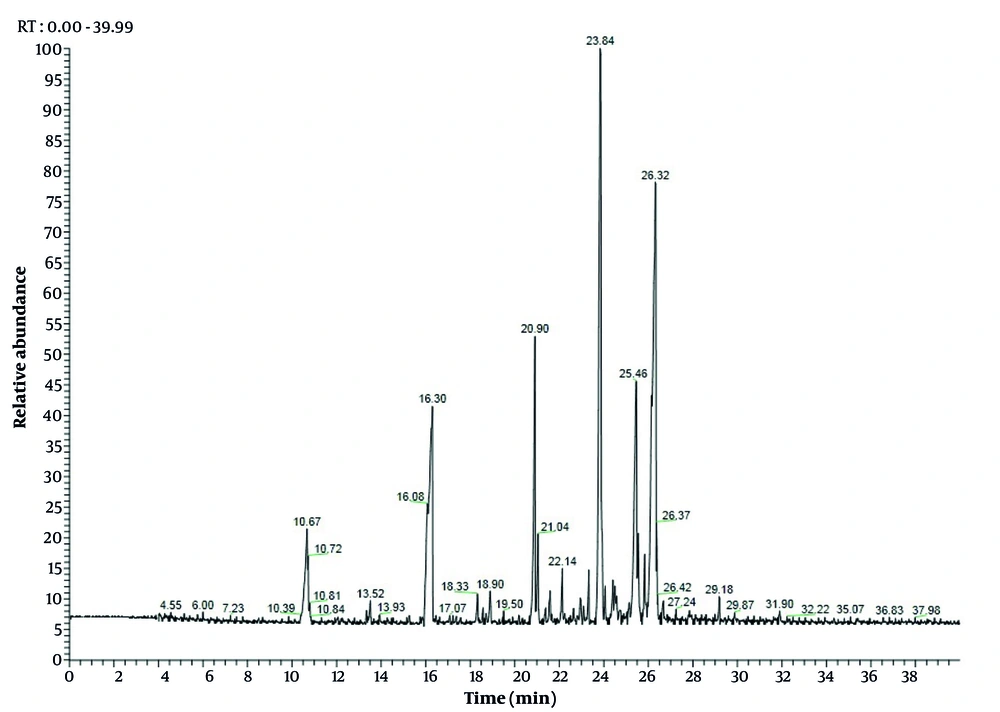
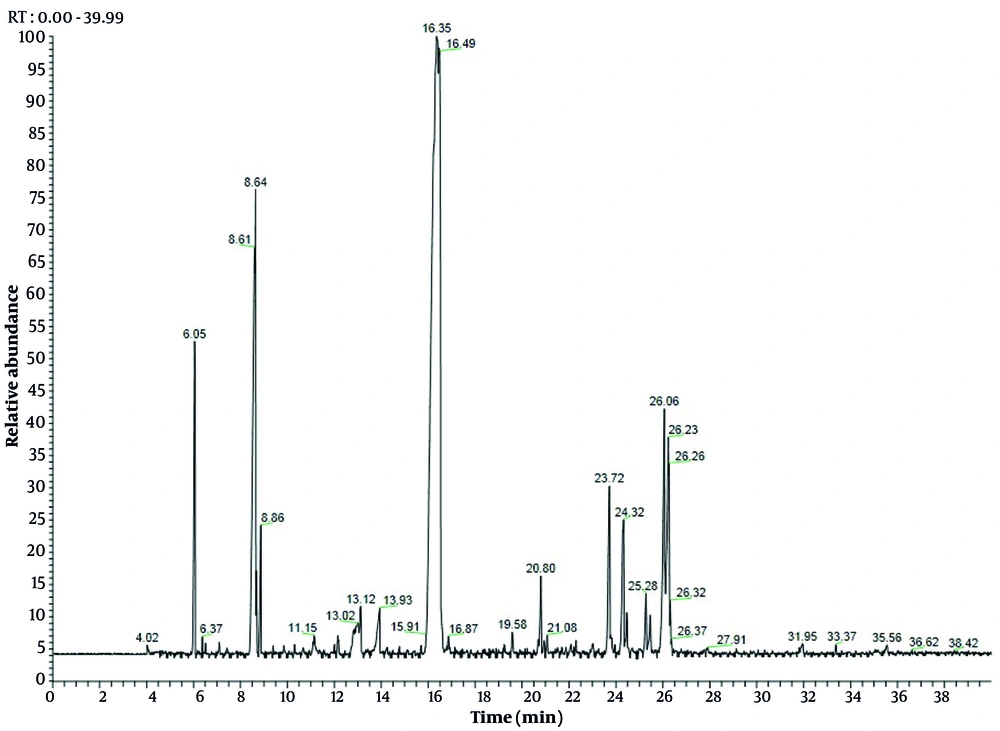
The EO of C. scabra was obtained and analyzed by GC and GC-MS. In total, 25 volatile constituents were identified, comprising an average of 98.89% and 98.91% of leaves and flowers, respectively (Table 2), mostly consisting of monoterpenoids and sesquiterpenoids. The percentage of oxygenated monoterpenes ranged from 27.02% to 57.97%, while oxygenated sesquiterpenes varied from 67.97% to 20.24% for leaves and flowers EO, respectively. Interestingly, the percentage of hydrocarbons (< 19%) was very low compared to terpenoids. These results align with findings from previous studies (2, 6, 11, 22). The leaves EO was dominated by α-bisabolol (22.92%), followed by spathulenol (19.66%), bornyl acetate (15.64%), acorenone B (9.66%), and O-cresol (6.79%) (Figure 1). Concurrently, flowers EO showed bornyl acetate (50.12%), O-cymene (12.77%), α-bisabolol (9.8%), and α-pinene (4.07%) as the major compounds (Figure 2). Previous studies have demonstrated that the chemical composition of EO from Cachrys species is quantitatively different but similar qualitatively (3, 22, 23). Pala-Paul et al. (2) revealed that the monoterpenes chemical group in all plant EO parts of C. trifida was higher than that of sesquiterpenes, with γ-terpinene, (E)-β-ocimene, limonene, p-cymene, α-pinene, and (Z)-β-ocimene as the main components of the aerial EO part, while the root EO contained terpinolene, γ-terpinene, and p-cymene. Conversely, in compounds obtained from the aerial parts of C. libanotis, C. alpina, and C. cristata, sesquiterpenes were higher than monoterpenes, with germacrene-D, γ-terpinene, p-cymene, caryophyllene oxide, and limonene as the most abundant ingredients (6, 11). Additionally, Bouderdara et al. (22) emphasized that Germacrene-D, a sesquiterpene hydrocarbon, was the most prevalent component in C. libanotis EO.
| Compounds | RT | % Area | KI a | ||
|---|---|---|---|---|---|
| Leaves | Flowers | Leaves | Flowers | ||
| α-pinene | - | 6.05 | ND | 4.07 ± 0.3 | 932 |
| Sabinene | - | 7.1 | ND | 0.79 ± 0.0 | 969 |
| O-cymene | - | 8.64 | ND | 12.77 ± 1.2 | 1022 |
| Z-β-ocimene | - | 8.86 | ND | 1.57 ± 0.1 | 1032 |
| O-cresol | 10.67 | 10.67 | 6.79 ± 0.3 | 0.41 ± 0.0 | 1050 |
| P-cresol | - | 11.15 | ND | 1.33 ± 0.0 | 1071 |
| Z-isocitral | - | 12.15 | ND | 0.61 ± 0.1 | 1160 |
| 3-thujanol | 13.52 | 13.12 | 1.58 ± 0.0 | 3.52 ± 0.2 | 1164 |
| ρ-cymene-8-ol | - | 13.93 | ND | 1.98 ± 0.2 | 1179 |
| Bornyl acetate | 16.3 | 16.35 | 15.64 ± 1.1 | 50.12 ± 1.3 | 1284 |
| Myrtenyl acetate | 18.33 | - | 3.01 ± 0.2 | ND | 1324 |
| 7-epi-sesquithujene | - | 19.58 | ND | 0.56 ± 0.0 | 1390 |
| Dictamnol | 18.9 | - | 2.64 ± 0.1 | ND | 1428 |
| Dehydro-sesquicineole | 20.9 | 20.8 | 5.6 ± 0.1 | 2.25 ± 0.3 | 1469 |
| Germacrene D | 21.04 | 21.08 | 1.61 ± 0.1 | 0.7 ± 0.1 | 1484 |
| Germacrene A | 21.59 | - | 2.32 ± 0.3 | ND | 1505 |
| δ-amorphene | 22.14 | - | 1.59 ± 0.1 | ND | 1511 |
| Elemol | 22.95 | - | 1.54 ± 0.1 | ND | 1548 |
| Caryophyllene oxide | 23.33 | - | 1.81 ± 0.0 | ND | 1582 |
| Spathulenol | 23.84 | 23.72 | 19.66 ± 1.3 | 3.67 ± 0.3 | 1577 |
| Cedrol | 24.41 | 24.32 | 1.21 ± 0.0 | 2.61 ± 0.2 | 1600 |
| 14-hydroxy-Z-caryophyllene | 24.49 | 24.46 | 1.31 ± 0.2 | 0.76 ± 0.1 | 1666 |
| 14-hydroxy-9-epi-E-caryophyllene | - | 25.28 | ND | 0.8 ± 0.2 | 1668 |
| α-bisabolol | 26.32 | 26.06 | 22.92 ± 1.1 | 9.8 ± 0.8 | 1685 |
| Acorenone B | 25.46 | 25.46 | 9.66 ± 0.8 | 0.59 ± 0.1 | 1697 |
| Total (%) | - | - | 98.89 | 98.91 | - |
| Monoterpene hydrocarbon | 0 | 4 | 0 | 19.2 | - |
| Oxygenated monoterpene | 4 | 6 | 27.02 | 57.97 | - |
| Sesquiterpene hydrocarbon | 2 | 2 | 3.93 | 1.5 | - |
| Oxygenated sesquiterpene | 10 | 7 | 67.94 | 20.24 | - |
Abbreviations: RT, retention time; ND, not detected; KI, Kovats Index.
a Kovats Index relative to C6 - C24 n-alkanes on the DB-5 column.
4.3. Antimicrobial Activity
As shown in Table 3, the highest MIC value was found for the petroleum ether extract against the growth of all tested bacterial strains, with values ranging from 5.00 to 10.00 mg/mL, except for the flower part on clinical P. aeruginosa, which showed low activity. Furthermore, the EOs and ethanol extract exhibited weak inhibitory effectiveness against the studied strains. Notably, flower EO was the only ingredient that showed mild activity against standard ATCC P. aeruginosa with an MIC value of 6.25 mg/mL. Overall, the MIC values presented from the extracts and EO are lower than those reported for the antibiotic used (ciprofloxacin). These results confirm that the significant antibacterial activity of C. scabra could be attributed to the presence of phenolics and flavonoids. Similar results were obtained from previous studies that demonstrated significant bactericidal activity when different extraction methods were used (9, 13, 21, 23, 24). Table 4 exhibits the fungicidal potential of EO and extracts of C. scabra on four Candida strains, viz., C. albicans, C. tropicalis, C. parapsilosis, and C. guilliermondii. Interestingly, the EOs exhibited strong inhibition on all Candida strains, with an inhibition range valued between 0.65 and 2.5 mg/mL. Additionally, mild-to-weak activity was observed for petroleum ether extracts, with values ranging between 6.25 and > 10 mg/mL. Based on our results, the EOs from leaves and flowers of C. scabra exhibited excellent antifungal activity against the growth of the studied fungi compared to all other treatments, even the antibiotic used (Ketoconazole). Similarly, Ozer et al. (23) and Koutsaviti et al. (24) demonstrated that Cachrys EO showed better antifungal activity than its extracts. Quite good results have also been obtained via EOs of different Cachrys spp., viz., C. cristata, C. microcarpos, and C. microcarpos against tested fungal strains (5).
| Extraction Type and Plant Part | Standard ATCC Staphylococcus aureus (mg/mL) | Standard ATCC Pseudomonas aeruginosa (mg/mL) | Clinical S. aureus (mg/mL) | Clinical P. aeruginosa (mg/mL) |
|---|---|---|---|---|
| Petroleum Ether | ||||
| Leaves | 10 | 5 | 5 | 5 |
| Flowers | 6.25 | 6.25 | 6.25 | > 10 |
| Ethanol | ||||
| Leaves | > 10 | > 10 | > 10 | > 10 |
| Flowers | > 10 | > 10 | > 10 | > 10 |
| EO | ||||
| Flowers | > 10 | 6.25 | > 10 | > 10 |
| Ciprofloxacin | 1.00 | 1.00 | 1.66 | 2.33 |
Abbreviation: EO, essential oil.
| Extraction Type and Plant Part | Candida albicans | Candida tropicalis | Candida parapsilosis | Candida guilliermondii |
|---|---|---|---|---|
| Petroleum Ether | ||||
| Leaves | 10 | 10 | 25 | 10 |
| Flowers | 6.25 | 12.5 | 6.25 | 12.5 |
| Ethanol | ||||
| Leaves | 25 | 25 | 25 | 25 |
| Flowers | 25 | 25 | 100 | 12.5 |
| EO | ||||
| Flowers | 1.25 | 2.5 | 0.62 | 1.25 |
Abbreviation: EO, essential oil.
4.4. Antioxidant Activity
All extracts obtained from plant parts exhibited weak antioxidant activity compared to ascorbic acid, with IC50 values ranging from 10 to 11 mg/mL. In contrast, the EO demonstrated higher radical scavenging activity, with IC50 values of 6.0 and 8.6 mg/mL for leaves and flowers, respectively (Table 5). Weak antioxidant capacity was also reported by previous studies (23). Correspondingly, the antioxidant activity of this genus demonstrated that the EOs possess a higher radical scavenging capability than crude extracts (7, 9, 12, 21). Aouachria et al. (9), as well as Menichini et al. (12), confirmed that the presence of functional groups like the hydroxyl group enhances antioxidant capability. Moreover, Marrelli et al. (8), along with Marrelli et al. (14), demonstrated that higher concentrations of EO correlate with increased antioxidant capability.
| Extraction Type and Plant Part | IC50 |
|---|---|
| Petroleum ether | |
| Leaves | 11 |
| Flowers | 10 |
| Ethanol | |
| Leaves | 10 |
| Flowers | 11 |
| EO | |
| Flowers | 8.6 |
| Leaves | 6 |
| Ascorbic acid | 0.014 |
4.5. Conclusions
A comparison of our results with other reports on the phytochemical and EO components and biological activities of C. scabra demonstrated considerable variation in chemical compositions and biological activities within this genus. Saponins and tannins were not found in our study. For EO, hydrocarbons were very low compared to terpenoids. The EO showed weak antibacterial activity but remarkable antifungal and antioxidant inhibition compared to herbal extracts, which can be attributed to its higher concentration of oxygenated terpenes and sesquiterpenes, such as bornyl acetate, α-bisabolol, and spathulenol. Further efforts are needed to evaluate the phytochemical profiles of C. scabra, as well as its biological properties, which may serve as a guide for selecting bio-medicinal materials of plant origin.