1. Context
One of the most serious medical and health issues in contemporary society, particularly in developing countries, is cancer (1). Among the most common malignant tumors in women, cervical cancer (CC) ranks fourth globally in terms of incidence and has a high mortality rate (2). Over the past 30 years, the proportion of young women diagnosed with CC has increased from 10% to 40% (3). Despite extensive research on the relationship between human papillomavirus (HPV) and CC, gaps remain regarding vaccination and prevention of this virus (4). These gaps are significant because the rising prevalence of HPV leads to increased cases of CC, posing serious risks and contributing to mortality among women worldwide.
The aim of this research is to address these gaps by closely examining the carcinogenic mechanisms of HPV, factors involved in HPV infection, and effective prevention and treatment methods for this virus. This study aims to enhance awareness within communities regarding CC and its association with HPV. Filling these gaps will facilitate the control of HPV prevalence through principled approaches.
2. Evidence Acquisition
This comprehensive review study examines the relationship between HPV and CC, with a particular focus on epidemiological patterns, molecular pathogenesis, and preventive strategies. We employed a narrative review methodology, which provides an optimal framework for synthesizing existing knowledge while incorporating recent advancements and addressing ongoing controversies in this well-established research domain.
Our literature search was conducted across multiple scientific databases, including PubMed, Scopus, Web of Science, and Google Scholar. The search strategy prioritized high-impact clinical studies, review articles, systematic reviews, meta-analyses, and consensus guidelines. Papers without a time limit until 2024 were included, ensuring that both foundational and the most current evidence up to 2024 were considered in our analysis. From an initial pool of identified publications, 63 studies meeting our inclusion criteria were selected for in-depth analysis.
The review encompasses several critical aspects of HPV-associated cervical carcinogenesis:
- Molecular mechanisms of HPV oncogenesis: Focus on viral persistence and the oncogenic effects of E6/E7 proteins.
- Global epidemiological patterns: Includes distribution of high-risk HPV types and geographic disparities in disease burden.
- Current screening modalities and prevention strategies: Covers Pap smears, HPV DNA testing, and vaccination.
- Emerging challenges in CC control: Addresses vaccine hesitancy and residual cancer risk in vaccinated populations.
The narrative review approach was specifically chosen to: (1) Provide a comprehensive synthesis of existing knowledge on HPV's role in CC development; (2) critically evaluate current prevention and treatment paradigms; and (3) identify key knowledge gaps requiring further investigation. This methodology allows for both breadth of coverage and depth of analysis, which is particularly valuable for understanding the complex interplay between viral factors, host responses, and clinical outcomes in HPV-related cervical pathology.
This evidence acquisition strategy ensures that our review incorporates the most current and relevant scientific findings while maintaining rigorous academic standards, ultimately providing a robust foundation for understanding HPV-driven cervical carcinogenesis and its clinical implications.
3. Results
3.1. Cervical Cancer
Cervical cancer is one of the most common cancers following breast cancer. Known risk factors for the development of CC include low socioeconomic status, smoking, early initiation of sexual activity, multiple sexual partners, multiple pregnancies, use of contraceptive medications, and infections with viruses such as HPV (5), human immunodeficiency virus (HIV) (6), herpes simplex virus type 2 (HSV-2) (7), and bacteria like Chlamydia trachomatis (8), Mycoplasma genitalium (9), and Trichomonas vaginalis (10). In most cases, CC arises from HPV infection, with the DNA of this virus identified in approximately 95% of malignant cervical lesions (11). Studies indicate that about 99.7% of diagnosed CC cases originate from intraepithelial lesions caused by HPV infection (12).
During the process of infecting cervical cells, HPV induces epigenetic changes, one of which is hypermethylation of genes (13). The cervical epithelial lining can develop various precancerous neoplasias known as cervical intraepithelial neoplasia (CIN). These include CIN 1, CIN 2, CIN 3, high-grade squamous intraepithelial lesions (HSIL), and low-grade squamous intraepithelial lesions (LSIL), with LSIL being the mildest form and CIN 2 categorized as moderate; CIN 3 represents the most severe disease state. The HSIL is the most dangerous precursor to CC, encompassing both CIN 2 and CIN 3; if left untreated, it poses a higher risk for cancer compared to LSIL or CIN 1 (14).
The time frame from initial HPV infection to persistent infection typically spans about seven years, which is the latency period for this disease. However, the complete onset of CC may take several decades (15).
3.2. Structural Details and Pathophysiology Related to Human Papillomavirus
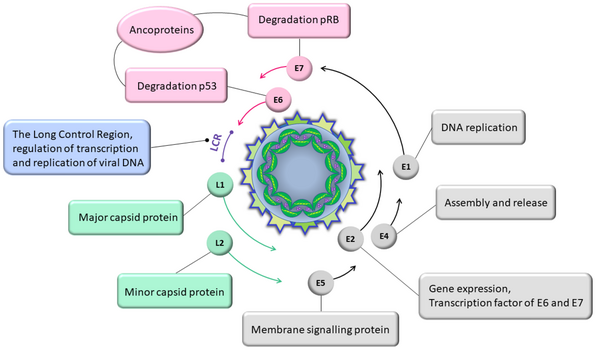
Human papillomavirus is a double-stranded DNA virus that affects both the nucleus and cytoplasm of superficial squamous cells, causing structural and morphological changes in these cells (16). The HPV genome is functionally divided into three regions.
3.2.1. Early Region
This includes open reading frames (ORFs) E1, E2, E4, E5, E6, and E7, which play roles in viral replication and carcinogenesis. Proteins E3, E5, and E8 were later identified, revealing that their genes are not uniformly expressed across HPV types (16-18).
3.2.2. Late Region
Composed of ORFs L1 and L2, this region codes for structural proteins and viral capsid proteins (both major and minor) (19). The HPV has approximately 360 molecules of the major capsid protein L1 and nearly 72 molecules of the minor capsid protein L2. L1 is responsible for binding HPV to the surface of host cells, while L2 is important for transferring the viral genome into the nucleus of target cells, where gene expression and viral DNA replication occur (20).
3.2.3. Long Control Region
Also known as the non-coding region (NCR) or upstream regulatory region (URR), this region encompasses about 10% of the viral genome and is located between the terminal end of ORF L1 and the start codon of E6. It contains the primary early promoter known as p97 and DNA replication regulatory elements, and it exhibits the highest prevalence of viral genome sequences (17) (Figure 1).
Sexual contact is one of the most important modes of HPV transmission between individuals. Other methods of HPV transmission in adults include transmission through contaminated water and the use of contaminated equipment in gynecological examination rooms. Additionally, vertical transmission from mother to newborn, as well as the presence of the virus's DNA in breast milk, amniotic fluid, and umbilical cord blood, are also routes through which the virus can be transmitted to infants (21).
3.3. Host Immune Response Against Human Papillomavirus and Human Papillomavirus Immune Evasion
According to research, before binding to keratinocytes (KCs), HPV attaches to heparan sulfate proteoglycans (HSPGs) present in certain areas of the basement membrane (22), leading to structural modifications in HPV, including cleavage of furin at the N-terminus of protein L2 (23). This facilitates the entry of the virus into the cell and its replication. Despite acting as a barrier against external factors and tumor growth, the basement membrane contains components like Laminin 332/Laminin 5, which are recognized as autocrine factors secreted by KCs into the extracellular matrix (ECM), promoting tumorigenesis (24).
Keratinocytes constitute a high percentage of the building blocks of the epidermis and act as non-professional antigen-presenting cells (APCs). They have the ability to secrete pro-inflammatory cytokines and chemokines, which can stimulate cluster of differentiation (CD) 4+ and CD8+ memory T-cells to secrete cytokines and activate cytotoxic functions (25). Natural killer (NK) cells play a crucial role in innate immune responses by combating virus-infected or tumor cells that lack major histocompatibility complex (MHC) molecules on their surface. Various immune evasion strategies exhibited by HPV can lead to immune tolerance and persistent HPV infection, resulting in cervical lesions and ultimately leading to CC (26).
The E5 protein can evade immune responses by reducing antigen presentation through limiting MHC-I transfer to the cell surface, which decreases recognition and detection of HPV-infected cells by CD8+ T-cells. Infected KCs with high-risk HPV lead to decreased expression of macrophage inflammatory protein-3α (MIP-3α) and subsequently inhibit Langerhans cells (LCs) migration (27). By inhibiting APC function, HPV prevents LC differentiation and migration, leading to suppression of acquired immune activity (28).
3.4. Pathogenesis of Cervical Cancer Development Following Human Papillomavirus Infection
For successful infection by HPV, attachment of the virus to host cells is essential. As an epitheliotropic virus, HPV infects epithelial cells through microtears created during unprotected or rough sexual intercourse or other sexually transmitted infections (STIs), ultimately leading to infection (29). The process of viral entry into host cells takes about 12 hours, likely due to structural changes in the capsid and host cell receptors (30). In contrast, intracellular movement is a very rapid process. Studies using virus-like particles (VLPs) have shown that HPVs tend to bind extensively to receptors on epithelial cell surfaces and eventually enter these cells. The function of VLPs composed solely of L1 and those composed of both L1 and L2 highlights L1's role as the primary attachment factor for host cells. Research using HPV-VLP has indicated that α6-integrin plays a major role as a primary receptor for HPV due to increased expression levels and enhanced binding of HPV to cells (31). Other receptors involved include annexin A2 and tetraspanin CD151 (32).
Various signaling events occur following viral binding to receptors that facilitate infection onset. The entry of HPV into cells is a diverse process dependent on viral type and occurs via endocytosis (33). Following endocytosis and under pH influence, capsid disassembly is observed in late endosomes/lysosomes. Lysosomal escape of the L2-DNA complex occurs through sorting nexin 17/SNX17 interactions mediated by L2 with various cellular sorting components (34). L2 directs viral DNA toward areas surrounding the host cell nucleus via retro-motor components such as dyneins. Entry into the host nucleus requires mitosis, which removes physical barriers between the cytosol and nucleoplasm while ultimately leading to disintegration of various Golgi apparatus components. Eventually, the L2-DNA complex gains access to the nucleus (35).
During carcinogenesis progression, the HPV genome initiates a complete life cycle and stability by integrating into the host genome. After inducing changes in the host genome, epithelial cells infected with HPV ultimately progress to CC (36) (Figure 2).
3.5. Diagnostic Methods for Human Papillomavirus Infection and Cervical Cancer
Through studies conducted via cervical cytology tests (Papanicolaou test/Pap test), it is possible to observe and microscopically study cells with normal shapes, minor abnormalities, and severe atypical abnormalities. Cells with minor abnormalities, known as atypical squamous cells of undetermined significance (ASC-US) and LSIL, indicate HPV infection; however, they do not definitively indicate precancerous lesions. The presence of cells with severe and unusual abnormalities, referred to as HSIL, confirms the existence of glandular and cancerous cells (37).
To identify active and oncogenic HPV infection, cervical samples are collected as part of primary HPV screening methods, which have higher sensitivity compared to cytology methods. These tests are more effective in identifying adenocarcinomas and their precursors (38). Other advantages of HPV testing (using DNA/RNA-based methods) include the ability to conduct long-term screening to identify high-risk HPV types that require a longer time to progress (39).
Immunohistochemistry (IHC) is a widely used method for identifying HPV in head and neck squamous cell carcinoma (HNSCC). In this type of infection, the neutralization of retinoblastoma protein (Rb) leads to overexpression of p16 (40). The identification of HPV infection requires the detection of HPV E6/E7 mRNA. For this purpose, in situ hybridization (ISH) and polymerase chain reaction (PCR) methods are commonly used (41). The ISH is employed as a specific method with high sensitivity for detecting high-risk HPV and is accompanied by the examination of viral DNA or RNA (42). Despite DNA-ISH having a possibility of false positive and negative results, RNA-ISH allows for direct observation of viral mRNA on formalin-fixed paraffin-embedded (FFPE) tissue samples, playing an important role in histopathological diagnosis (43).
In PCR methods, selective amplification of a portion of viral DNA leads to an increase in the production and replication of double-stranded HPV DNA sequences, allowing for the observation, examination, and diagnosis of HPV infection and CC. Over the past 20 years, ultrasound has become a common method for diagnosing CC, as it is faster, cheaper, and more accessible compared to other imaging techniques. Transrectal and transvaginal ultrasound provide accurate images of cervical tumors because the probe is positioned close to the tumor, enabling precise imaging (44).
Usually, sophisticated instruments are used to diagnose this malignancy. However, many of these techniques are time-consuming, expensive, and intrusive, and they are only available in major hospital labs. CC and precancerous lesions are mostly diagnosed by visual inspection in nations with low resources. However, it might be difficult to visually examine the ectocervical and endocervical areas for tiny lesions (e.g., Pap smear approach). Furthermore, these lesions could go unnoticed and develop into aggressive malignancy if tests are spaced out (45).
There is no formal program for CC screening in many low- and middle-income nations, and conventional techniques like cervical cytology are not available because of inadequate infrastructure (46). Among the main reasons why women are reluctant to be tested are fear of discomfort from the insertion of a speculum, embarrassment in conservative communities, concern about negative findings, difficulty of access to healthcare facilities in remote regions, lack of time, and financial restrictions. Many individuals are diagnosed with CC at advanced stages, which makes treatment more challenging, and these factors lead to the late detection of the illness (47).
3.6. Prevention and Treatment
To prevent CC, screening tests such as the Pap smear conducted by gynecologists and the administration of the HPV vaccine are highly effective (48). Gardasil and Cervarix are the most commonly used HPV vaccines approved and registered by the Food and Drug Administration (FDA) (49). The quadrivalent Gardasil vaccine provides 100% protection against low-risk types (the cause of most genital warts), while the nine-valent Gardasil vaccine protects against nine HPV types (two low-risk and seven high-risk types). Studies indicate that Cervarix, used exclusively for women, provides significant protection against two high-risk types, HPV16 and HPV18.
Other treatment options for CC include surgery (simple hysterectomy or partial removal of the cervix), radiation therapy, and chemotherapy (50).
3.7. Vaccines
Receiving the HPV vaccine is an effective method for preventing infection with the virus and inhibiting the development of precancerous lesions in the genital tract (51). The L1 protein is the major capsid protein in HPV, constituting 80% of the viral capsid proteins (52). One of the primary strategies to prevent HPV-related cervical cancer (CxCa) is the development of preventive vaccines using the L1 protein. Currently, three highly effective L1-based vaccines approved by the FDA are Cervarix, Gardasil, and Gardasil 9 (53).
Cervarix is a bivalent HPV vaccine (2vHPV) that protects against HPV16/18 infections. Gardasil is a quadrivalent HPV vaccine (4vHPV) effective against HPV6/11/16/18. Compared to Cervarix, the additional genotypes covered by Gardasil are responsible for approximately 90% of genital warts (54, 55). Gardasil also reduces HPV infections in various body areas, including the anus, vulva, penis, and oral cavity (56). Gardasil 9 (9vHPV) targets HPV-6/11/16/18/31/33/45/52/58 (57). These three vaccines exhibit high safety and efficacy, preventing approximately 70% to 90% of HPV-related cancers (51).
The VLPs, composed of the L1 viral protein produced through recombinant technology, play a key role in eliciting immune responses. This process generates antibodies that protect the body against future infections (58). The 4vHPV vaccine is effective in preventing genital warts, precancerous or dysplastic lesions, and CC in both males and females aged 9 to 26 years. The 9vHPV vaccine is approved for preventing genital warts, precancerous or dysplastic lesions, and cancers of the cervix, vulva, vagina, and anus in males and females aged 9 to 45 years (55).
Compared to bivalent vaccines, the quadrivalent vaccine also prevents genital warts, as it includes L1-VLPs of HPV6 and HPV11, which are the most common causes of skin papillomas. The nonavalent vaccine, compared to the bivalent one, has increased the concentration of L1-VLPs for HPV-16 and HPV-18 (59). Additionally, three new vaccines — Cecolin, Walrinvax, and Cervavax — have recently been licensed in China and India (54).
If 75% of adolescents, both girls and boys, receive the HPV vaccine, it is possible to eradicate HPV-16 in the general population. Although males exhibit a weak antibody response to natural infection, they show a strong immune response to VLP vaccines, with nearly 100% seroconversion. HPV vaccines are recommended for boys aged 11 to 21 years to prevent anal cancer and genital warts (60). The Gardasil vaccine provides 90% protection against various HPV types, genital warts, and anal epithelial neoplasia, which may lead to anal cancer in young men (61). Furthermore, this vaccine has demonstrated good efficacy in males aged 16 to 26 years, significantly reducing the prevalence of HPV-related diseases (62).
4. Conclusions
The vast majority (over 99%) of CC cases result from persistent infection with specific strains of HPV, particularly HPV16 and HPV18. These high-risk HPV strains can cause precancerous changes in cervical cells that may progress to CC over time if left untreated. It can be stated that HPV infection is the primary cause of nearly all CC cases. HPV testing can detect the presence of high-risk HPV strains and facilitate early identification of women at risk for developing CC. When administered before initial HPV infection, HPV vaccines are highly effective in preventing CC. Regular screening for CC and timely treatment of precancerous cervical lesions are crucial for preventing the development of invasive CC.
In summary, there is a strong causal relationship between persistent HPV infection and CC, underscoring the importance of HPV vaccination, CC screening, and prompt treatment of precancerous changes to reduce the burden of this highly preventable type of cancer.