Abstract
Objectives:
Due to the anti-inflammatory effects of dextrose prolotherapy, we evaluated the effectiveness of extra-articular, neurofascial dextrose prolotherapy in chronic ankle ligament injury.Methods:
Patients with chronic ankle ligament injury entered this uncontrolled before-after study based on eligibility criteria. Patients who consented to participate in the study filled out the prepared questionnaire containing demographic data, the Cumberland ankle instability tool (CAIT), and the Visual Analogue Scale (VAS). The initial CAIT score of less than 25 indicated functional instability following an ankle sprain. Patients underwent neurofascial prolotherapy with dextrose 12.5%. Two injections within one month were done. The CAIT was completed one, three, and six months after the intervention.Results:
Twenty-five patients with chronic ankle ligament injury were investigated. The mean CAIT score was 1.88 (± 2.35) before the intervention, which increased significantly over the study (P < 0.001). The CAIT score reached 21.84 (± 6.04) in the sixth month after the intervention. Moreover, the VAS score decreased significantly over the study from 6.12 (± 2.35) before the intervention to 1.24 (± 0.43) in the sixth month after the intervention (P < 0.001).Conclusions:
Our findings revealed the therapeutic effectiveness of dextrose neurofascial prolotherapy in decreasing pain and functional instability in patients suffering chronic ankle pain due to ligamentous injury accompanied by chronic ankle instability.Keywords
Ankle Ligament Injury Neurofascial Dextrose Prolotherapy CAIT Questionnaire
1. Background
Chronic ankle ligament injury is a common problem in musculoskeletal clinics (1, 2). Chronic ankle instability is a sequela of repeated sprains; however, it can happen after a single sprain, even after six years (3). Various methods have been introduced to treat ligamentous injuries (4, 5), such as corticosteroid injection (6), platelet-rich plasma, prolotherapy (7), and even botulinum toxin injection (8) but the most effective treatment of ankle tendinopathy requires further investigations. Proliferation therapy, also named prolotherapy, is known as an effective treatment of different musculoskeletal disorders such as rotator cuff tendinopathy, plantar fasciitis, and knee osteoarthritis (9-11).
Although enthesofascial/intra-articular and myofascial prolotherapy has been used widely, neurofascial or perineural prolotherapy is a different concept introduced by Pybus (12). Neurofascial prolotherapy fights neurogenic inflammation developed by chronic constriction injury (CCI) made by peripheral nerves penetrating the fascia. Orthodromic C pain fiber impulses and antidromic substance P releases (by inflammation of Nervi Nervorum) can cause pain, swelling, and inflammation of soft tissues. Dextrose can decrease neurogenic inflammation by binding to presynaptic calcium channels (13). In addition, extra-articular injection is easier and safer to perform, decreasing the side effects of intra-articular injection like infection and bleeding.
Hyperosmolar dextrose solution as a proliferant is used to repair and restore soft tissues. We have used this technique for rotator cuff tendinopathy and knee osteoarthritis (7, 14).
2. Objectives
Due to the beneficial effects of neurofascial dextrose prolotherapy, in this study, we evaluated the effectiveness of extra-articular neurofascial dextrose prolotherapy in chronic ankle ligament injury.
3. Methods
We performed an uncontrolled before-after clinical trial in the Research Center of Clinical Biomechanics and Ergonomics at AJA University of Medical Sciences. This study was registered with the registration number IRCT20180416039323N2 and ethically approved by the Institutional Review Board of AJA University of Medical Sciences.
Patients with chronic ankle ligament injury referred to the Physical Medicine and Rehabilitation Department of Imam Reza Hospital entered the study based on the eligibility criteria. The patients were aged 20 to 75 years with a history of ankle sprain or injury whose magnetic resonance imaging confirmed ligamentous injury, with no pain and disability improvement after conservative treatments. Patients with a history of the rheumatologic disorder, diabetes, immune deficiency, or recent bone fracture or infection around the ankle were excluded. Patients were also excluded if they were on anticoagulant agents or had any previous allergic reaction to dextrose or lidocaine injection. Patients who consented to participate in the study filled out the prepared questionnaire containing demographic data, the Cumberland ankle instability tool (CAIT), and the Visual Analogue Scale (VAS) for pain.
The CAIT questionnaire is a self-declared questionnaire containing nine questions measuring functional ankle instability of patients with at least one period of an ankle sprain. The maximum score is 30 points, and lower scores indicate more instability. The initial score of less than 25 (≤ 25) indicates functional instability following ankle sprain with 96.6% sensitivity and 86.6% specificity. The minimal clinically significant difference for the CAIT questionnaire is ≥ 3 points (7, 15). We used the Persian version of the CAIT questionnaire validated by Haji-Maghsoudi et al. among Iranian athletes with a lateral ankle sprain (16). In this study, 46 athletes who had experienced at least one episode of lateral ankle sprain entered the study and completed the Persian version of the CAIT questionnaire. The Cronbach's alpha was 0.64, and the test-retest correlation coefficient for the total score of CAIT was 0.95. The VAS is a 0 to 10 measure representing the subjective pain level: from 0, "no pain" to 10, the "worst pain."
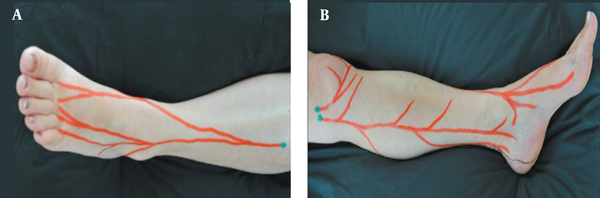
Based on our previous study of chronic rotator cuff tendinopathy (7), patients underwent neurofascial prolotherapy with 12.5% dextrose solution (1 to 3 ratios of 50% dextrose and 2% lidocaine solutions). The Hackett-Hemwall technique similarly used 15% dextrose and 0.2% lidocaine solution (17). The patient laid supine while the ankle was placed on the bed in a resting position to avoid any traction on ligaments. Tender points were identified by palpating the superficial peroneal and saphenous nerve as explained by Waldman and shown in Figure 1 (18). In addition, tender points on ligaments and bony structures around the ankle were identified. All areas around the ankle were sterilized with 10% iodine solution. A 25-gauge, 3.5-cm needle was used to inject superficial tender points, and deeper structures were injected using sonographic guidance. In each tender point, 1 to 2 cc of the solution was injected to a total volume of 10 cc.
We did not use any intra-articular injection. Two injections within one month were done. After each injection, patients were recommended using ice and relative rest and were followed for any immediate or latent adverse reaction. They were asked to stop any other treatment during the study and allowed to use acetaminophen as needed. Any intensive exercise or prolonged weight-bearing was restricted. All previous exercise-based treatments continued. The outcomes were measured one, three, and six months after the intervention.
A, superficial peroneal; and B, saphenous nerve branches demonstrating chronic constriction injury sites explained by Waldman (18).
Data are presented as the mean and standard deviation for continuous variables and numbers and proportions for categorical variables. The baseline and post-intervention data normality was examined with the Kolmogorov-Smirnov test. Significant differences in the VAS and CAIT scores at the follow-up were tested with repeated measures ANOVA. Levene's test investigated the homogeneity of variances. The significance level was set at 0.05. All data analyses were performed with IBM SPSS version 26 for Windows.
4. Results
This study enrolled 25 patients with chronic ankle ligament injury (23 females to two males). The mean age and Body Mass Index (BMI) of the patients were 58.64 (± 8.69) years and 29.09 (± 3.77), respectively. The ANCOVA showed that the age and BMI were not significant covariates for the VAS score [F (1,22) = 1.03, P = 0.321 and F (1,22) = 3.77, P = 0.06, respectively] and CAIT score [F (1,22) = 0.04, P = 0.83 and F (1,22) = 0.01, P = 0.89, respectively].
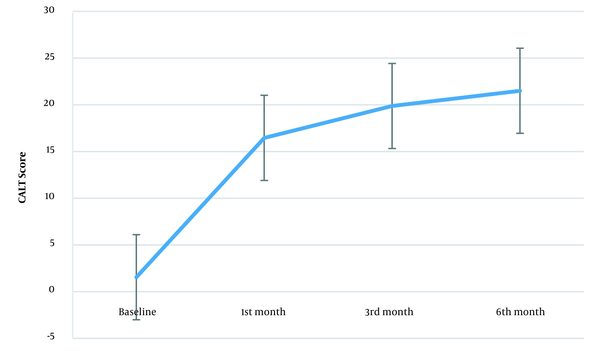
Figure 2 shows the CAIT score trend during the study. The mean CAIT score was 1.88 (± 2.35) before the intervention. This score was 16.45 (± 6.24), 20.28 (± 5.76), and 21.84 (± 6.04) in the first, third, and sixth months after the intervention, respectively. The repeated measures ANOVA assessed the within-subject effect of prolotherapy during the study (prolotherapy × time). Mauchly’s test showed that the sphericity assumption was violated (W = 0.332, P < 0.001). The Greenhouse-Geisser estimates of sphericity for correcting the degree of freedom were used (ɛ = 0.735), showing that time was a significant factor [F (2.20, 50.73) = 122.90, P < 0.001]. In the first, third, and sixth months after the intervention, the mean CAIT score differences were more than the minimal clinically significant difference (≥ 3) (Table 1). The effect size showed the considerable effect of prolotherapy on the CAIT score (2.32, 6.84, and 3.18 in the first, third, and sixth months after the intervention, respectively).
Changes in the mean CAIT score over the study. Error bars represent the 95% CI (CAIT, Cumberland ankle instability tool; CI, confidence interval).
Within-Group Analysis of Changes in VAS and CAIT Scores
| Variables and Features | CAIT Score | VAS Score |
|---|---|---|
| First month-baseline | ||
| Mean difference a | 14.91 | -2.84 |
| t-statistic (DoF) | 11.38 | -15.81 (24) |
| P-value | < 0.001 | < 0.001 |
| Third month-baseline | ||
| Mean difference | 18.40 | -4.24 |
| t-statistic (DoF) | 15.97 | -35.49 (24) |
| P-value | < 0.001 | < 0.001 |
| Sixth month-baseline | ||
| Mean difference | 19.96 | -4.88 |
| t-statistic (DoF) | 15.90 | -40.66 (24) |
| P-value | < 0.001 | < 0.001 |
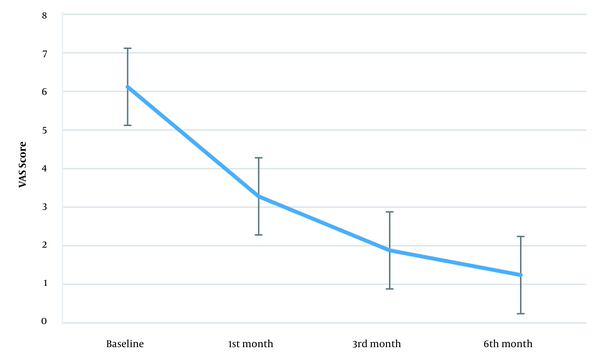
Figure 3 also shows the VAS score trend. The baseline VAS score was 6.12 (± 0.33) while this measure was 3.28 (± 0.79) in the first, 1.88 (± 0.44) in the third, and 1.24 (± 0.43) in the sixth month after the intervention. Mauchly's sphericity test showed the sphericity assumption violation (W = 0.535, P = 0.01). After Greenhouse-Geisser correction (ɛ = 0.717), time showed a significant effect on the VAS score [F (2.15, 163) = 461.69, P < 0.001]. Also, the mean VAS score differences in the first, third, and sixth months after the intervention were significant compared to baseline (Table 1), and the effect size was significant (3.16, 7.09, and 8.12 in the first, third, and sixth months after the intervention, respectively).
Changes in the mean VAS score over the study. Error bars represent the 95% CI (VAS, Visual Analog Scale; CI, confidence interval).
5. Discussion
This study indicated that neurofascial prolotherapy significantly improves pain and functional instability in chronic ankle ligament injury. The VAS and CAIT scores decreased significantly after one month, and this trend continued till the sixth month after the intervention. The increased CAIT score was much more than the minimal clinically significant difference. The study patients had severe ankle instability and experienced different therapies without sufficient pain relief. Although this study was a clinical trial, its strength is questioned due to the lack of a control group.
Pain and functional instability are essential complications of ankle sprains, reported in 50 to 79% of patients (19). Although performing surgery to stabilize the joint is an option, postoperative pain is still a significant problem in 13 to 35% of patients (20). Non-operative treatment options have short-term and insufficient effects on pain and instability (17). A systematic review of the dextrose prolotherapy effect on chronic musculoskeletal pain concluded that prolotherapy helps manage tendinopathies, osteoarthritis, and pain in the spine or pelvis due to ligament injuries (10, 21). Nevertheless, dextrose neurofascial prolotherapy is a novel approach targeting chronic constriction injury, resulting in neurogenic inflammation (22, 23). Different studies have revealed the pain-reducing effect of neurofascial dextrose injection, which fights against this neurogenic inflammation pathway. Lyftogt studies since 2005 have shown its therapeutic effects on Achilles tendinopathy, knee, shoulder, elbow, and low back pain (24, 25). We showed the therapeutic effect of neurofascial dextrose prolotherapy on rotator cuff tendinopathy in another study with a method explained by Waldman (18).
After an acute injury, inflammatory mediators like prostaglandins, interleukins, nerve growth factors, and even tumor necrosis factors can modify the receptor potential of the nervous system, which is predominantly done on C fibers, leading to chronic pain (26). This continuous production and release of pain-producing neuropeptides like substance P and calcitonin gene-related peptide (CGRP) is the main characteristic of chronic pain. Studies have shown that this process occurs in the absence of leukocytes (22).
Our study was the first interventional study that assessed the effect of neurofascial prolotherapy on ankle pain. In a similar study, Hauser et al. showed the beneficial effect of dextrose prolotherapy on chronic ankle pain (17). Hauser et al.’s study used a similar injection approach but focused on injecting tender points around ankle ligaments and bony structure. Although this study is retrospective and the weak methodological approach impels us to the cautious interpretation of results, its main finding showed a reduction in the pain level. In Hauser et al.'s study, the VAS score dropped from 7.9 to 1.6, similar to our study (6.12 to 1.24). Decreased stiffness, crepitation, medication use, and even anxiety were the other findings not measured in our study.
This interventional study is limited by its uncontrolled design. We did not compare our intervention with a control group, thus a possible source of bias. The symptom severity in our patients did not allow us to use other nonsurgical approaches. Our patients tried other non-invasive treatments without symptom improvement, but neurofascial prolotherapy significantly reduced their pain and functional instability. Other studies with controlled interventions need to evaluate the efficacy of neurofascial prolotherapy in a better way.
5.1. Conclusion
To our knowledge, this is the first interventional study to evaluate the efficacy of dextrose neurofascial prolotherapy in chronic ankle ligament injury. Our findings revealed the therapeutic effectiveness of dextrose neurofascial prolotherapy in decreasing pain and functional instability in patients suffering chronic ankle pain due to ligamentous injury accompanied by chronic instability. Despite the limitations of our study protocol, we recommend this procedure as a non-invasive, effective way to alleviate such patients’ symptoms.
References
-
1.
Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37(1):73-94. [PubMed ID: 17190537]. https://doi.org/10.2165/00007256-200737010-00006.
-
2.
Waterman BR, Belmont PJ, Cameron KL, Deberardino TM, Owens BD. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38(4):797-803. [PubMed ID: 20145281]. https://doi.org/10.1177/0363546509350757.
-
3.
Noor N, Urits I, Degueure A, Rando L, Kata V, Cornett EM, et al. A Comprehensive Update of the Current Understanding of Chronic Fatigue Syndrome. Anesth Pain Med. 2021;11(3). e113629. [PubMed ID: 34540633]. [PubMed Central ID: PMC8438707]. https://doi.org/10.5812/aapm.113629.
-
4.
Dabir S, Mosaffa F, Hosseini B, Alimoradi V. Comparison of the Combined Femoral and Lateral Femoral Cutaneous Nerve Block Plus Popliteal Block with Spinal Anesthesia for Thigh Tourniquet Pain During Foot or Ankle Surgery: A Randomized Clinical Trial. Anesth Pain Med. 2020;10(4). e103674. [PubMed ID: 33134147]. [PubMed Central ID: PMC7539047]. https://doi.org/10.5812/aapm.103674.
-
5.
Tarbiat M, Majidi M, Manouchehrian N. Frequent Spinal Anesthesia in a Patient with Traumatic Lower Extremity Injury: A Case Report. Anesth Pain Med. 2019;9(2). e88595. [PubMed ID: 31341827]. [PubMed Central ID: PMC6614781]. https://doi.org/10.5812/aapm.88595.
-
6.
Rezasoltani Z, Najafi S, Azizi S, Forough B, Maleki N, Fateh HR. The comparison of Shock Wave therapy and Corticosteroid injection on the treatment of Plantar Fasciitis. Ann Mil Health Sci. 2013;11(1):53-60.
-
7.
Kazempour Mofrad M, Rezasoltani Z, Dadarkhah A, Kazempour Mofrad R, Abdorrazaghi F, Azizi S. Periarticular Neurofascial Dextrose Prolotherapy Versus Physiotherapy for the Treatment of Chronic Rotator Cuff Tendinopathy: Randomized Clinical Trial. J Clin Rheumatol. 2021;27(4):136-42. [PubMed ID: 32975923]. https://doi.org/10.1097/RHU.0000000000001218.
-
8.
Rezasoltani Z, Dadarkhah A, Tabatabaee SM, Abdorrazaghi F, Kazempour Mofrad M, Kazempour Mofrad R. Therapeutic Effects of Intra-articular Botulinum Neurotoxin Versus Physical Therapy in Knee Osteoarthritis. Anesth Pain Med. 2021;11(3). e112789. [PubMed ID: 34540630]. [PubMed Central ID: PMC8438713]. https://doi.org/10.5812/aapm.112789.
-
9.
Imani F, Hejazian K, Kazemi MR, Narimani-Zamanabadi M, Malik KM. Adding Ozone to Dextrose and Somatropin for Intra-articular Knee Prolotherapy: A Randomized Single-Blinded Controlled Trial. Anesth Pain Med. 2020;10(5). e110277. [PubMed ID: 34150571]. [PubMed Central ID: PMC8207853]. https://doi.org/10.5812/aapm.110277.
-
10.
Chung MW, Hsu CY, Chung WK, Lin YN. Effects of dextrose prolotherapy on tendinopathy, fasciopathy, and ligament injuries, fact or myth?: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99(46). e23201. [PubMed ID: 33181700]. [PubMed Central ID: PMC7668443]. https://doi.org/10.1097/MD.0000000000023201.
-
11.
Imani F, Patel VB. Therapeutic Challenges for Knee Osteoarthritis. Anesth Pain Med. 2019;9(3). e95377. [PubMed ID: 31497526]. [PubMed Central ID: PMC6712428]. https://doi.org/10.5812/aapm.95377.
-
12.
Why W. Neural prolotherapy. Patients. 2011;3(2):639-43.
-
13.
Shen YP, Li TY, Chou YC, Ho TY, Ke MJ, Chen LC, et al. Comparison of perineural platelet-rich plasma and dextrose injections for moderate carpal tunnel syndrome: A prospective randomized, single-blind, head-to-head comparative trial. J Tissue Eng Regen Med. 2019;13(11):2009-17. [PubMed ID: 31368191]. https://doi.org/10.1002/term.2950.
-
14.
Rezasoltani Z, Taheri M, Mofrad MK, Mohajerani SA. Periarticular dextrose prolotherapy instead of intra-articular injection for pain and functional improvement in knee osteoarthritis. J Pain Res. 2017;10:1179-87. [PubMed ID: 28553139]. [PubMed Central ID: PMC5439936]. https://doi.org/10.2147/JPR.S127633.
-
15.
Wright CJ, Linens SW, Cain MS. Establishing the Minimal Clinical Important Difference and Minimal Detectable Change for the Cumberland Ankle Instability Tool. Arch Phys Med Rehabil. 2017;98(9):1806-11. [PubMed ID: 28137476]. https://doi.org/10.1016/j.apmr.2017.01.003.
-
16.
Haji-Maghsoudi M, Naseri N, Nouri-Zadeh S, Jalayi S. [Evidence of reliability for persian version of the “Cumberland Ankle Instability Tool (CAIT)” in Iranian athletes with lateral ankle sprain]. Arch Rehabil. 2016;16(4):304-11.
-
17.
Hauser R, Hauser M, Cukla J. Dextrose prolotherapy injection for chronic ankle pain. Pract Pain Manag. 2010;10(1):70-6.
-
18.
Waldman SD. Pain Management. Amsterdam: Elsevier Health Sciences; 2011.
-
19.
Al Adal S, Pourkazemi F, Mackey M, Hiller CE. The Prevalence of Pain in People With Chronic Ankle Instability: A Systematic Review. J Athl Train. 2019;54(6):662-70. [PubMed ID: 31184959]. [PubMed Central ID: PMC6602397]. https://doi.org/10.4085/1062-6050-531-17.
-
20.
Ahn BH, Cho BK. Persistent Pain After Operative Treatment for Chronic Lateral Ankle Instability. Orthop Res Rev. 2021;13:47-56. [PubMed ID: 33907476]. [PubMed Central ID: PMC8064723]. https://doi.org/10.2147/ORR.S299409.
-
21.
Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A Systematic Review of Dextrose Prolotherapy for Chronic Musculoskeletal Pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139-59. [PubMed ID: 27429562]. [PubMed Central ID: PMC4938120]. https://doi.org/10.4137/CMAMD.S39160.
-
22.
Lyftogt J. Prolotherapy and Achilles tendinopathy: a prospective pilot study of an old treatment. Australasian Musculoskeletal Medicine. 2005;10(1).
-
23.
Ghasemi M, Mosaffa F, Hoseini B, Behnaz F. Comparison of the Effect of Bicarbonate, Hyaluronidase, and Lidocaine Injection on Myofascial Pain Syndrome. Anesth Pain Med. 2020;10(3). e101037. [PubMed ID: 32944559]. [PubMed Central ID: PMC7472162]. https://doi.org/10.5812/aapm.101037.
-
24.
Lyftogt J. 313 Prolotherapy for Achilles tendinopathy: The best solution? Journal of Science and Medicine in Sport. 2007;10(Suppl 1). https://doi.org/10.1016/s1440-2440(07)70321-3.
-
25.
Reeves D, Rabago D. Therapeutic injection of dextrose: Prolotherapy, perineural injection therapy and hydrodissection. Illinois, USA: PM&R Knowledge Now; 2019, [cited 2021]. Available from: https://now.aapmr.org/therapeutic-injection-of-dextrose-prolotherapy-perineural-injection-therapy-and-hydrodissection/.
-
26.
Malik KM, Beckerly R, Imani F. Musculoskeletal Disorders a Universal Source of Pain and Disability Misunderstood and Mismanaged: A Critical Analysis Based on the U.S. Model of Care. Anesth Pain Med. 2018;8(6). e85532. [PubMed ID: 30775292]. [PubMed Central ID: PMC6348332]. https://doi.org/10.5812/aapm.85532.