Abstract
Background:
General anesthesia induces endocrine, immunologic, and metabolic responses. Anesthetic drugs affect the endocrine system by changing the level of stress hormones and hemodynamic variables of the patient.Objectives:
The purpose of this study was to compare the effects of propofol and dexmedetomidine on hemodynamic parameters and stress-induced hormones in laparoscopic cholecystectomy (LC) surgery.Methods:
Seventy patients of elective LC were included in this study. The patients were randomly assigned into two equal groups of propofol (75 µg/kg/min) and dexmedetomidine (0.5 µg/kg/hour) as anesthesia maintenance. Hemodynamic parameters (heart rate and mean atrial pressure), blood sugar, and serum epinephrine level were monitored and recorded from pre-anesthesia period to 10 min after entry to post-anesthesia care unit (PACU) according to a planned method.Results:
Heart rate and mean atrial pressure changes were significantly lower in dexmedetomidine group in all stages compared to propofol group (P < 0.001). Also, the rises in blood glucose and serum epinephrine levels in the dexmedetomidine group were significantly higher than in the propofol group (P < 0.001).Conclusions:
Anesthesia maintenance by dexmedetomidine showed a significant difference in hemodynamic parameters in comparison with propofol. While dexmedetomidine had better effects on controlling hemodynamic parameters, propofol showed better effects on decreasing stress hormones, and it can be suggested for LC surgery.Keywords
Hemodynamic Parameters Dexmedetomidine Laparoscopic Cholecystectomy Propofol Stress Hormones
1. Background
Recently, among multiple surgical techniques for the treatment of cholelithiasis and cholecystitis, laparoscopic cholecystectomy (LC) is a method of choice (1, 2). Both neuraxial and general anesthesia are common anesthetic techniques in these patients. Various physiological changes may occur during anesthesia for laparoscopic surgery, which may lead to hemodynamic instability, increase in intra-abdominal pressure caused by pneumoperitoneum (CO2 insufflation), and patient position (3). Following the painful irritations that occur after the induction of anesthesia and during surgery, the patient’s stress responses are triggered. Hence, choosing a method that minimizes the hormonal fluctuations caused by stress responses will be desirable (4). Today, one of the serious concerns of researchers and physicians is to find effective medications to control the increase of inflammatory and stress responses. Epinephrine is a neurotransmitter of the endogenous catecholamines group, which causes an increase in heart rate, vasoconstriction, and dilatation of airways (5).
Propofol generates the most pronounced decrease in systemic blood pressure compared with other anesthetics, which, in addition to rapid anesthetic effects and lower side effects, has a faster anesthesia return than other intravenous anesthetics (6-8); it is also known as the best anesthetic drug for continuous infusion due to its rapid metabolism and the lack of cumulative effects. After the induction of anesthesia, propofol may result in a decrease in blood pressure and bradycardia due to less inhibition of the parasympathetic nervous system compared to sympathetic one.
The use of preoperative α2 receptor agonists improves hemodynamic stability due to its numerous beneficial effects, including analgesic effects, inhibition of sympathetic outputs, anti-anxiety properties, and reduction of norepinephrine levels (9). They protect myocardial muscle because of positive effects on myocardial oxygen supply and cardiac oxygen demand (10-13). Stress response involves various hormones, such as release of epinephrine, cortisol, some cytokines like interleukin, TNF, and growth factors, and complement system activation. This stress response to surgery can be reduced by activating an alpha-dual adrenergic receptor (14, 15). Dexmedetomidine is a highly selective α2 receptor agonist with 1600-fold affinity to α1 receptor (16). The use of dexmedetomidine before anesthesia has a positive effect on hemodynamic stability, which has been associated with reduced postoperative mortality and reduction of unpleasant postoperative complications (17-20). The results of laboratory and clinical studies showed that dexmedetomidine reduces inflammatory responses (21, 22), and animal studies also showed inhibition of pro-inflammatory cytokines (23). In addition, the results of in vitro studies on whole human blood samples reported suppression of lipopolysaccharides, which produce pro-inflammatory mediators including TNF-α, interleukin-6, and IL-8 (24, 25). Besides vasodilatory effects, alpha-2 agonists have sympathetic suppressive, sedative, and hypnotic effects (26). Alpha-2 agonists, such as clonidine, have a blood pressure lowering effect, and therefore reduce surgical bleeding (16, 27); in addition, dexmedetomidine facilitates analgesia and anesthesia in humans and reduces the severity of postoperative pain and nausea (28-30). This drug is approved for use for up to 24 hours at a maximum dose of 0.7 micrograms per kilogram of body weight per hour (31). Several studies showed that dexmedetomidine is relatively safe even after long-term administration in high doses, with few side effects (32). Intravenous dexmedetomidine reduces the need to use high dose of propofol administration to achieve and maintain desirable bispectral index score (BIS) with low side effect (33).
2. Objectives
Considering the beneficial effects of propofol and dexmedetomidine, we aimed to study their effects on the identical BIS, hemodynamic parameters, and inflammatory and stress factors in patients undergoing LC surgery.
3. Methods
3.1. Study Design
After approving the research in the Ethics Committee of Jundishapur University of Medical Sciences of Ahvaz (code: IR.AJUMS.REC.1398.778) and obtaining an informed consent from the patients, the study was conducted as a double-blind randomized clinical trial (patient and bio-statistician were blinded). According to the criteria by the American Society of Anesthesiology (ASA) class I-II, we included 75 patients (age range: 20 - 60 years) undergoing elective LC surgery. Patients were divided into two equal groups: propofol (n = 34) and dexmedetomidine (n = 34).
Common standard monitoring measurements, including electrocardiogram (ECG), blood pressure, pulse-oximetry, end-tidal CO2 capnometry (Model M3B Edan), and BIS (Cerebral State Monitor, Model CSM 2Danmeter A/S) were performed upon the entry of patients to the operating room. Demographic information (age, sex, height, weight) and changes in hemodynamic parameters (mean atrial pressure [MAP], heart rate) and end-tidal CO2 were recorded. The patients were oxygenated at a rate of 5 liters per minute with 100% oxygen for 3 min. All patients received a combination of midazolam (0.05 mg/kg; Chemidarou Iran Co.), fentanyl (2 μg/kg; Aburaihan Iran Co.), sodium thiopental (4 mg/kg; Trittau, Germany), and atracurium (0.5 mg/kg; Caspian Tamin) for induction and morphine (0.1 mg/kg; Darupakhsh Iran Co.) was administered for analgesia after induction. The maintenance of anesthesia in propofol group was infusion of propofol at a dose of 75 μg/kg/min (Dongkook pharm. co. ltd, Korea), and in the dexmedetomidine group was infusion of dexmedetomidine at a dose of 0.5 μg/kg/hour (Exir Co. ltd Iran). Atracurium (0.1 mg/kg) was used alternately every 20 min as a relaxant (34). The depth of anesthesia in both groups was maintained between 45 and 50 BIS through the infusion of propofol and dexmedetomidine. Both groups received remifentanil (0.7 µg/kg/min; intravenous [IV] after intubation; Fresenius SE & Co KgaA, India) until the end of surgery.
This study was carried out at Golestan Hospital of Ahvaz Jundishapur University of Medical Sciences, Iran, from January 2020 to February 2021.
3.2. Study Patients
In this study, a total of 75 consecutive patients scheduled for elective LC were enrolled (Figure 1). Inclusion criteria were: not having urgent/emergency surgery, obesity grade II or III (BMI > 35), chronic liver disease, diabetes, renal failure, endocrine problems (pheochromocytoma), rheumatic disease, cardiovascular disease or malignancy, and egg allergy; patients receiving drugs with known effects on sympathetic response or hormonal secretion such as beta blockers, epinephrine, and insulin; patients consuming benzodiazepines, dextrose serum, and dexmedetomidine; and pregnant or lactating mothers. Exclusion criteria were: unwillingness to participate in the study and changing the method of surgery to classical open cholecystectomy.
Consort flow chart of study
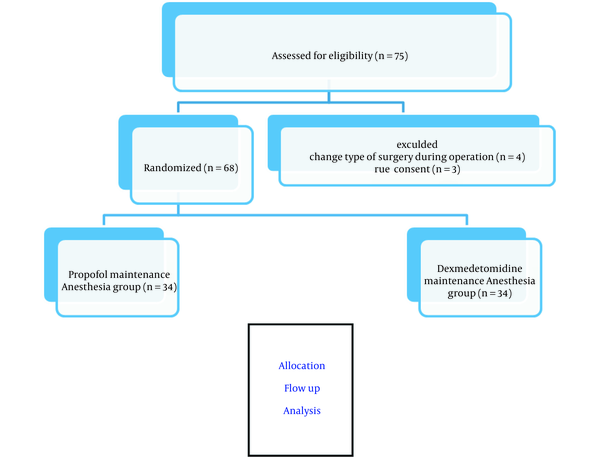
3.3. Study Variables
Demographic data (age, sex, and BMI) and duration of anesthesia and surgery were recorded. Patients’ hemodynamic parameters, including heart rate (HR) and MAP, were recorded during the following stages of the study: pre-induction, induction time, and intra-operatively every 5 min till the end of anesthesia and 10 min after PACU entry.
Plasma level of epinephrine and blood glucose (BS) had been measured immediately after induction and at the end of surgery (before reversal of neuromuscular block).
3.4. Laboratory Measurements
Before and after each surgery, blood samples were obtained, and plasma was prepared. Epinephrine level was measured by human epinephrine ELISA kit (Taoyuan, Taiwan; Catalog Number: KA1877), with 18 to 6667 pg/mL normal value range. Glucose was measured by a biochemical colorimetric method (Karaj, Iran, Parsazmon; Catalog Number: G45215)
3.5. Statistical Analysis
Categorical variables were reported as percentages, and continuous variables were reported as the mean ± standard deviation or median (interquartile range). The assumption of normality was evaluated using the Shapiro–Wilk or Kolmogorov–Smirnov test. Epinephrine and glucose levels were compared using the analysis of variance (ANOVA) and Tukey’s post-hoc tests. HR and MAP were analyzed by repeated measures ANOVA (RM-ANOVA) test. Data were analyzed using GraphPad prism® software version 8.3.0.
4. Results
A total of 68 patients (n = 34 in each group) were studied. The median age was 54 years in the propofol group (median + IQR, 54, 49 - 58) and was 56 years in the dexmedetomidine group (median + IQR, 56, 48 - 59). In propofol group, 58% of the patients (n = 20) and in dexmedetomidine group, 69.58% of the patients (n = 24) were females. The demographic data are listed in Table 1. As can be seen, weight was higher in the dexmedetomidine group (P = 0.019), but there was no significant difference in other variables between the two groups.
| Variables | Propofol (n = 34) | Dexmedetomidine (n = 34) | P-Value |
|---|---|---|---|
| Gender | 0.621 | ||
| Female | 20 (58) | 24 (69.57) | |
| Male | 14 (42) | 10 (30.43) | |
| Age (y) IQR | 54 (47 - 57) | 56 (46 - 60) | 0.360 |
| Height (cm) | 165.8 ± 5.15 | 166.94 ± 5.51 | 0.857 |
| Weight (kg) | 67.9 ± 6.06 | 72.88 ± 7.50 | 0.019* |
Epinephrine level in the different times of immediately after induction and end of surgery, in propofol and dexmedetomidine groups, was (mean ± SD, 95.38 ± 8.14 ,104.1 ± 9.87, 95.74 ± 9.94, 114.0 ± 14.58, respectively). ANOVA test showed a significant difference between all times and agents (P < 0.0001) (Figure 2A).
Changes immediately after induction and end of surgery in two groups under maintenance of anesthetic propofol (P, n = 34) and dexmedetomidine (D, n = 34) administration. (A) Plasma level Kruskal–Wallis test analysis (P < 0.0001), (*, ** and ***P < 0.0001). (B) Plasma level of glucose ANOVA analysis (P < 0.0001), (*, ** and *** P < 0.0001)
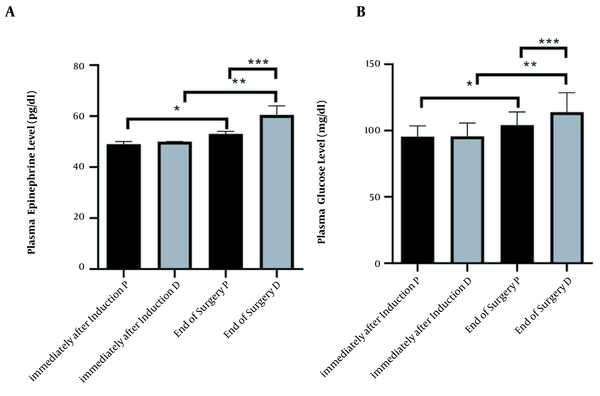
Glucose level in the different times immediately after induction and end of the surgery, in propofol and dexmedetomidine groups was (mean ± SD, 49.06 ± 1.04, 53.50 ± 3.73, 49.71 ± 1.21, 61.38 ± 3.70, respectively). ANOVA test showed a significant difference between all times and agents (P < 0.0001) (Figure 2B).
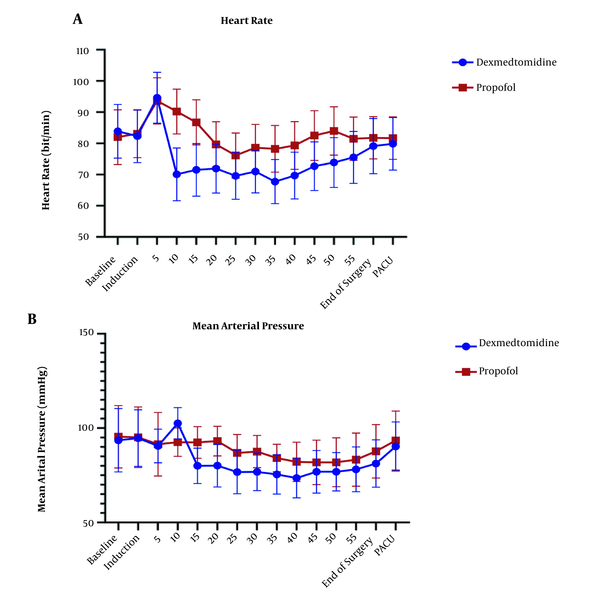
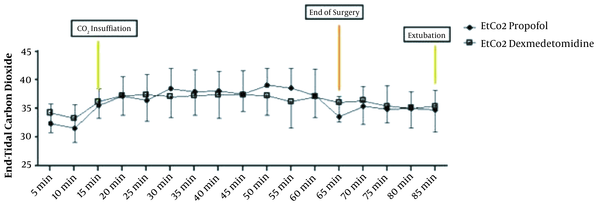
The repeated measure ANOVA test for analysis effect of time and agent showed significant difference in HR and MAP (P < 0.001) and no significant difference in EtCO2 (P = 0.636) (Figures 3 and 4).
(A) Changes in heart rate (HR) in the P group (n = 34,) and the D group (n = 34). Preoperative (-20), operative, and postoperative (PACU) measurements. P < 0.001 (Repeated measure ANOVA). (B) Changes in mean arterial pressure (MAP) in the P group (n = 34) and the D group (n = 34). Preoperative (-20), operative, and postoperative (PACU) measurements. P < 0.001 (Repeated measure ANOVA)
End-tidal CO2 form intubation till extubation in two groups of study (P = 0.693)
5. Discussion
5.1. Epinephrine and Glucose
Although surgical techniques have been developed, general stress response is an inseparable part of surgery (35). This stress is induced by surgical incision and is a complex of reactions that happen in the site of incision, such as increases in cAMP, epinephrine, and other catecholamines (36). Reduction of physiologic stress response is an anesthesiology goal for maintaining a patient under stable hemodynamic level during the surgery. In molecular view, this response induces biochemical changes, including epinephrine, norepinephrine, cortisol, ACTH, IL1, and IL6 rise, which are detectable in the blood as the central compartment of the body (34). Epinephrine is a rational index in surgical stress. In comparison to conventional cholecystectomy, LC has a low stress response (Table 2) (37, 38). According to the study by Glaser et al., in different types of cholecystectomy surgery (classic versus LC) catecholamine changes has the same trend (39). In this study, epinephrine was selected as a rational index, and glucose was measured as an indirect marker of changes in catecholamines. Although epinephrine levels increased in both groups, the propofol group had significantly low-stress profile than the dexmedetomidine group. The trend of glucose level changes was similar to the epinephrine trend. Dexmedetomidine, as the only anesthetic agent, did not have an appropriate protective effect on stress hormones (40). This may be due to a different pharmacodynamic mechanism compared to propofol. Dexmedetomidine induces deep sleep via noradrenergic locus ceruleus neuron hyperpolarization compared to agonistic action on GABAa receptors pathway by propofol (41, 42).
Previous Studies
| Type of Surgery | Researcher | Year of Publication | Variables Measured | Anesthesia Methods | Results | References |
|---|---|---|---|---|---|---|
| Laparoscopic surgery | ||||||
| Cholecystectomy | Glaser et al. | 1995 | The level N, E, ACTH, Cortisol LC versus CC | Mixed | LC ↓ in stress response versus CC | (37) |
| Non- cholecystectomy | Marana et al. | 2010 | N, E, ACTH, Cortisol, GH, PRL, TSH, FT3, FT4 | Induction: STP; Maintenance: Propofol vs. sevoflurane | In propofol group level E ↓, Sevoflurane group: E ↑ | (43) |
| Azemati et al. | 2013 | CRP, Glucose, cortisol, HR. MAP | Maintenance: Propofol vs. isoflurane | In propofol group level E ↓, Isoflurane group: E ↑ | (44) | |
| Non- laparoscopic surgery | ||||||
| Not define type of surgery | Adams et al. | 1994 | N, E, ACTH, Cortisol, ADH, HR and arterial pressure was measured | Induction: STP and Propofol; Maintenance: Propofol vs isoflurane | Level EPI in propofol group significantly was lower | (45) |
| Ihn et al. | 2009 | N, E, ACTH, Cortisol, Glucose and Il-6 | Induction: STP; Maintenance: Propofol vs. sevoflurane | Level E and Glucose ↑ in sevoflurane; Level E and Glucose in propofol ↑ and is lower than sevofluarne | (46) | |
| Bulow et al. | 2007 | Cortisol, Blood sugar | TIVA by propofol; Comparison: DEX vs remifentany | Level BS and cortisol was higher in DEX group | (47) |
5.2. Hemodynamic Parameters
The HR level had a statistically significant difference in all stages of measurement after induction in the two groups. After induction of anesthesia, the groups had the opposite trend, dexmedetomidine has declining trend of HR changes, but propofol has increased this trend. Trend confusion in 25-35 minute after induction could explain by surgery manipulation. In a non-major type of surgery study by chattopadhyay et al. (41), dexmedetomidine group had lower HR in comparison to propofol, which was consistent with the results of the present study. In a study by Azemati et al. (44), after induction, the mean HR significantly decreased in both propofol groups compared to baseline, and remained below baseline until the end of the surgery, which had the same results as our study.
The mean of MAP has shown a statistically significant difference (P > 0.05) in both groups and had lower amount in dexmedetomidine group, that back to mechanism of alpha 2 agonist characteristic of dexmedetomidine. Many studies showed dexmedetomidine alone or as adjuvant could control blood pressure (41, 47-49). Tilvawala et al. (50) compared the effect of propofol and sevoflurane on hemodynamic changes in laparoscopic surgeries; they reported that there was no significant difference between the two groups in hemodynamic changes. In a study by Azameti et al., arterial blood pressure was significantly reduced in both propofol and isoflurane groups, which is consistent with the results of this study, in which only the propofol receiving group had a significantly lower MAP at the end of surgery (44). In the propofol group, the mean of MAP at the end of surgery was significantly lower than baseline, and in the isoflurane group, this factor was not significantly different from baseline (51). After the end of the surgery, the mean blood glucose in the patients receiving propofol was significantly lower than that in the dexmedetomidine group, and dexmedetomidine group had higher amount in glucose level at the end of surgery point in comparison itself in immediately after induction points.
In a study by Azemati et al., glucose was significantly increased in both groups receiving propofol and isoflurane, but one hour after cutting but one hour after incision of the surgeon and one hour after the end of the surgery, the amount of glucose decreased significantly in propofol group (44).
At the end of the surgery, the mean epinephrine plasma levels in patients receiving propofol were significantly lower, and the increase in epinephrine levels in the dexmedetomidine group was significantly higher than that in the propofol group.
5.3. Conclusions
Although epinephrine level was low in propofol group, there was a significant difference in hemodynamic parameters that suggest future studding on the combination of dexmedetomidine propofol. Consistent hemodynamic and lack of significant difference in important factors could refer to monitoring depth anesthesia by BIS.
References
-
1.
Longo MA, Cavalheiro BT, de Oliveira Filho GR. Laparoscopic cholecystectomy under neuraxial anesthesia compared with general anesthesia: Systematic review and meta-analyses. J Clin Anesth. 2017;41:48-54. [PubMed ID: 28802605]. https://doi.org/10.1016/j.jclinane.2017.06.005.
-
2.
Norozi V, Ghazi A, Amani F, Bakhshpoori P. Effectiveness of Sublingual Buprenorphine and Fentanyl Pump in Controlling Pain After Open Cholecystectomy. Anesth Pain Med. 2021;11(3). e113909. [PubMed ID: 34540635]. [PubMed Central ID: PMC8438705]. https://doi.org/10.5812/aapm.113909.
-
3.
Hayden P, Cowman S. Anaesthesia for laparoscopic surgery. CEACCP. 2011;11(5):177-80. https://doi.org/10.1093/bjaceaccp/mkr027.
-
4.
McCarthy C, Saldova R, Wormald MR, Rudd PM, McElvaney NG, Reeves EP. The role and importance of glycosylation of acute phase proteins with focus on alpha-1 antitrypsin in acute and chronic inflammatory conditions. J Proteome Res. 2014;13(7):3131-43. [PubMed ID: 24892502]. https://doi.org/10.1021/pr500146y.
-
5.
Wehrwein EA, Joyner MJ. Regulation of blood pressure by the arterial baroreflex and autonomic nervous system. Handb Clin Neurol. 2013;117:89-102. [PubMed ID: 24095118]. https://doi.org/10.1016/B978-0-444-53491-0.00008-0.
-
6.
Yamaura K, Hoka S, Okamoto H, Kandabashi T, Akiyoshi K, Takahashi S. Changes in left ventricular end-diastolic area, end-systolic wall stress, and fractional area change during anesthetic induction with propofol or thiamylal. J Anesth. 2000;14(3):138-42. [PubMed ID: 14564580]. https://doi.org/10.1007/s005400070021.
-
7.
Oriby ME, Elrashidy A. Comparative Effects of Total Intravenous Anesthesia with Propofol and Remifentanil Versus Inhalational Sevoflurane with Dexmedetomidine on Emergence Delirium in Children Undergoing Strabismus Surgery. Anesth Pain Med. 2021;11(1). e109048. [PubMed ID: 34221936]. [PubMed Central ID: PMC8236675]. https://doi.org/10.5812/aapm.109048.
-
8.
Soltani F, Tabatabaei S, Jannatmakan F, Nasajian N, Amiri F, Darkhor R, et al. Comparison of the Effects of Haloperidol and Dexmedetomidine on Delirium and Agitation in Patients with a Traumatic Brain Injury Admitted to the Intensive Care Unit. Anesth Pain Med. 2021;11(3). e113802. [PubMed ID: 34540634]. [PubMed Central ID: PMC8438711]. https://doi.org/10.5812/aapm.113802.
-
9.
Bahr MH, Rashwan DAE, Kasem SA. The Effect of Dexmedetomidine and Esmolol on Early Postoperative Cognitive Dysfunction After Middle Ear Surgery Under Hypotensive Technique: A Comparative, Randomized, Double-blind Study. Anesth Pain Med. 2021;11(1). e107659. [PubMed ID: 34221933]. [PubMed Central ID: PMC8236574]. https://doi.org/10.5812/aapm.107659.
-
10.
Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci Lett. 2012;529(1):70-4. [PubMed ID: 22917606]. https://doi.org/10.1016/j.neulet.2012.08.008.
-
11.
Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2013;64(4):301-7. [PubMed ID: 23646238]. [PubMed Central ID: PMC3640161]. https://doi.org/10.4097/kjae.2013.64.4.301.
-
12.
J NS, Kumar S, Vijay T. To Compare the Efficacy of Dexmedetomidine Versus Labetalol in Providing Controlled Hypotension in Functional Endoscopic Sinus Surgery. Anesth Pain Med. 2021;11(1). e108915. [PubMed ID: 34221935]. [PubMed Central ID: PMC8241463]. https://doi.org/10.5812/aapm.108915.
-
13.
Asri S, Hosseinzadeh H, Eydi M, Marahem M, Dehghani A, Soleimanpour H. Effect of Dexmedetomidine Combined with Inhalation of Isoflurane on Oxygenation Following One-Lung Ventilation in Thoracic Surgery. Anesth Pain Med. 2020;10(1). e95287. [PubMed ID: 32309196]. [PubMed Central ID: PMC7145426]. https://doi.org/10.5812/aapm.95287.
-
14.
Kang SH, Kim YS, Hong TH, Chae MS, Cho ML, Her YM, et al. Effects of dexmedetomidine on inflammatory responses in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2013;57(4):480-7. [PubMed ID: 23240685]. https://doi.org/10.1111/aas.12039.
-
15.
Yacout AG, Osman HA, Abdel-Daem MH, Hammouda SA, Elsawy MM. Effect of intravenous dexmedetomidine infusion on some proinflammatory cytokines, stress hormones and recovery profile in major abdominal surgery. Alexandria J Med. 2019;48(1):3-8. https://doi.org/10.1016/j.ajme.2011.11.001.
-
16.
Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127(15):1576-84. [PubMed ID: 23513068]. [PubMed Central ID: PMC3979354]. https://doi.org/10.1161/CIRCULATIONAHA.112.000936.
-
17.
Imani F, Farahmand Rad R, Salehi R, Alimian M, Mirbolook Jalali Z, Mansouri A, et al. Evaluation of Adding Dexmedetomidine to Ropivacaine in Pediatric Caudal Epidural Block: A Randomized, Double-blinded Clinical Trial. Anesth Pain Med. 2021;11(1). e112880. [PubMed ID: 34221950]. [PubMed Central ID: PMC8241816]. https://doi.org/10.5812/aapm.112880.
-
18.
Margulis R, Francis J, Tischenkel B, Bromberg A, Pedulla D, Grtisenko K, et al. Comparison of Dexmedetomidine and Dexamethasone as Adjuvants to Ultra-Sound Guided Interscalene Block in Arthroscopic Shoulder Surgery: A Double-Blinded Randomized Placebo-Controlled Study. Anesth Pain Med. 2021;11(3). e117020. [PubMed ID: 34540645]. [PubMed Central ID: PMC8438728]. https://doi.org/10.5812/aapm.117020.
-
19.
Kumar CM, Chua AWY, Imani F, Sehat-Kashani S. Practical Considerations for Dexmedetomidine Sedation in Adult Cataract Surgery Under Local/Regional Anesthesia: A Narrative Review. Anesth Pain Med. 2021;11(4). e118271. [PubMed ID: 34692445]. [PubMed Central ID: PMC8520679]. https://doi.org/10.5812/aapm.118271.
-
20.
Ibrahim IM, Hassan R, Mostafa RH, Ibrahim MA. Efficacy of Dexmedetomidine Infusion Without Loading Dose on Hemodynamic Variables and Recovery Time During Craniotomy: A Randomized Double-blinded Controlled Study. Anesth Pain Med. 2021;11(2). e113410. [PubMed ID: 34336625]. [PubMed Central ID: PMC8314083]. https://doi.org/10.5812/aapm.113410.
-
21.
Totonchi Z, Rezvani H, Ghorbanloo M, Yazdanian F, Mahdavi M, Babaali N, et al. Effect of dexmedetomidine infusion on hemodynamics and stress responses in pediatric cardiac surgery: A randomized trial. Growth Factors. 2016;3:4.
-
22.
Ghasemi M, Behnaz F, Hajian H. The Effect of Dexmedetomidine Prescription on Shivering during Operation in the Spinal Anesthesia Procedures of Selective Orthopedic Surgery of the Lower Limb in Addicted Patients. Anesth Pain Med. 2018;8(2). e63230. [PubMed ID: 30009149]. [PubMed Central ID: PMC6035481]. https://doi.org/10.5812/aapm.63230.
-
23.
Li B, Li Y, Tian S, Wang H, Wu H, Zhang A, et al. Anti-inflammatory Effects of Perioperative Dexmedetomidine Administered as an Adjunct to General Anesthesia: A Meta-analysis. Sci Rep. 2015;5:12342. [PubMed ID: 26196332]. [PubMed Central ID: PMC4508837]. https://doi.org/10.1038/srep12342.
-
24.
Kawasaki T, Kawasaki C, Ueki M, Hamada K, Habe K, Sata T. Dexmedetomidine suppresses proinflammatory mediator production in human whole blood in vitro. J Trauma Acute Care Surg. 2013;74(5).
-
25.
Malayeri AR, Hemmati AA, Arzi A, Rezaie A, Ghafurian-Boroojerdnia M, Khalili HR. A Comparison of the Effects of Quercetin Hydrate With Those of Vitamin E on the Levels of IL-13, PDGF, TNF-α, and INF-γ in Bleomycin-Induced Pulmonary Fibrosis in Rats. Jundishapur J Nat Pharm Prod. 2016;11(2). https://doi.org/10.17795/jjnpp-27705.
-
26.
El-Tahan MR, Mowafi HA, Al Sheikh IH, Khidr AM, Al-Juhaiman RA. Efficacy of dexmedetomidine in suppressing cardiovascular and hormonal responses to general anaesthesia for caesarean delivery: a dose-response study. Int J Obstet Anesth. 2012;21(3):222-9. [PubMed ID: 22681971]. https://doi.org/10.1016/j.ijoa.2012.04.006.
-
27.
Janatmakan F, Nesioonpour S, Javaherforoosh Zadeh F, Teimouri A, Vaziri M. Comparing the Effect of Clonidine and Dexmedetomidine on Intraoperative Bleeding in Spine Surgery. Anesth Pain Med. 2019;9(1). e83967. [PubMed ID: 30881906]. [PubMed Central ID: PMC6408748]. https://doi.org/10.5812/aapm.83967.
-
28.
Osman M, Al-Medani S, Beltagi R, El-Sawy M, Neemat-Allah F. Effects of dexmedetomidine versus morphine on surgical stress response and analgesia in postoperative open cardiac surgery. Res Opin Anesth Intensive Care. 2015;1(1). https://doi.org/10.4103/2356-9115.161316.
-
29.
Janatmakan F, Nassajian N, Jarirahmadi S, Tabatabaee K, Zafari M. Comparison of the Effect of Dexmedetomidine and Remifentanil on Pain Control After Spinal Surgery: A Double-Blind, Randomized Clinical Trial. Anesth Pain Med. 2021;11(2). e111533. [PubMed ID: 34336614]. [PubMed Central ID: PMC8314072]. https://doi.org/10.5812/aapm.111533.
-
30.
Oriby ME. Comparison of Intranasal Dexmedetomidine and Oral Ketamine Versus Intranasal Midazolam Premedication for Children Undergoing Dental Rehabilitation. Anesth Pain Med. 2019;9(1). e85227. [PubMed ID: 30881910]. [PubMed Central ID: PMC6412317]. https://doi.org/10.5812/aapm.85227.
-
31.
El Mourad MB, Elghamry MR, Mansour RF, Afandy ME. Comparison of Intravenous Dexmedetomidine-Propofol Versus Ketofol for Sedation During Awake Fiberoptic Intubation: A Prospective, Randomized Study. Anesth Pain Med. 2019;9(1). e86442. [PubMed ID: 30881913]. [PubMed Central ID: PMC6412910]. https://doi.org/10.5812/aapm.86442.
-
32.
Ueki M, Kawasaki T, Habe K, Hamada K, Kawasaki C, Sata T. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69(7):693-700. [PubMed ID: 24773263]. https://doi.org/10.1111/anae.12636.
-
33.
Mirkheshti A, Memary E, Nemati Honar B, Jalaeefar A, Sezari P. Local Adjuvant Dexmedetomidine Effect on the Dose of Sedation During Awake Endotracheal Intubation. J Police Med. 2016;5(2):97-102.
-
34.
Sawyers JL. Current status of conventional (open) cholecystectomy versus laparoscopic cholecystectomy. Ann Surg. 1996;223(1):1-3. [PubMed ID: 8554409]. [PubMed Central ID: PMC1235056]. https://doi.org/10.1097/00000658-199601000-00001.
-
35.
Akhondzadeh R, Olapour A, Rashidi M, Elyasinia F. Comparison of Sedation with Dexmedetomidine Alfentanil Versus Ketamine-Alfentanil in Patients Undergoing Closed Reduction of Nasal Fractures. Anesth Pain Med. 2020;10(4). e102946. [PubMed ID: 33134144]. [PubMed Central ID: PMC7539046]. https://doi.org/10.5812/aapm.102946.
-
36.
Madsen SN, Fog-Moller F, Christiansen C, Vester-Andersen T, Engquist A. Cyclic AMP, adrenaline and noradrenaline in plasma during surgery. Br J Surg. 1978;65(3):191-3. [PubMed ID: 205303]. https://doi.org/10.1002/bjs.1800650315.
-
37.
Glaser F, Sannwald GA, Buhr HJ, Kuntz C, Mayer H, Klee F, et al. General stress response to conventional and laparoscopic cholecystectomy. Ann Surg. 1995;221(4):372-80. [PubMed ID: 7726672]. [PubMed Central ID: PMC1234586]. https://doi.org/10.1097/00000658-199504000-00007.
-
38.
Albooghobeish M, Ghomeishi A, Adarvishi S, Neisi A, Mahmoodi K, Asadi M, et al. The Effect of Preoperative Zintoma Capsule on Postoperative Nausea and Vomiting After Laparoscopic Cholecystectomy. Anesth Pain Med. 2018;8(5). e67132. [PubMed ID: 30533389]. [PubMed Central ID: PMC6241159]. https://doi.org/10.5812/aapm.67132.
-
39.
Glaser F, Kuntz C, Sannwald GA, Mayer H, Herfarth C. General stress response in laparoscopic and conventional cholecystectomy. Swiss surgery. 1996;1:41-4.
-
40.
Hadipourzadeh F, Mousavi S, Heydarpur A, Sadeghi A, Ferasat-Kish R. Evaluation of the Adding Paracetamol to Dexmedetomidine in Pain Management After Adult Cardiac Surgery. Anesth Pain Med. 2021;11(3). e110274. [PubMed ID: 34540629]. [PubMed Central ID: PMC8438704]. https://doi.org/10.5812/aapm.110274.
-
41.
Chattopadhyay U, Mallik S, Ghosh S, Bhattacharya S, Bisai S, Biswas H. Comparison between propofol and dexmedetomidine on depth of anesthesia: A prospective randomized trial. J Anaesthesiol Clin Pharmacol. 2014;30(4):550-4. [PubMed ID: 25425783]. [PubMed Central ID: PMC4234794]. https://doi.org/10.4103/0970-9185.142857.
-
42.
Imani F, Zaman B, De Negri P. Postoperative Pain Management: Role of Dexmedetomidine as an Adjuvant. Anesth Pain Med. 2020;10(6). e112176. [PubMed ID: 34150582]. [PubMed Central ID: PMC8207883]. https://doi.org/10.5812/aapm.112176.
-
43.
Marana E, Colicci S, Meo F, Marana R, Proietti R. Neuroendocrine stress response in gynecological laparoscopy: TIVA with propofol versus sevoflurane anesthesia. J Clin Anesth. 2010;22(4):250-5. [PubMed ID: 20522354]. https://doi.org/10.1016/j.jclinane.2009.07.011.
-
44.
Azemati S, Savai M, Khosravi MB, Allahyari E, Jahanmiri F. Combination of remifentanil with isoflurane or propofol: effect on the surgical stress response. Acta Anaesthesiol Belg. 2013;64(1):25-31. [PubMed ID: 23767174].
-
45.
Adams HA, Schmitz CS, Baltes-Gotz B. [Endocrine stress reaction, hemodynamics and recovery in total intravenous and inhalation anesthesia. Propofol versus isoflurane]. Anaesthesist. 1994;43(11):730-7. German. [PubMed ID: 7840401]. https://doi.org/10.1007/s001010050115.
-
46.
Ihn CH, Joo JD, Choi JW, Kim DW, Jeon YS, Kim YS, et al. Comparison of stress hormone response, interleukin-6 and anaesthetic characteristics of two anaesthetic techniques: volatile induction and maintenance of anaesthesia using sevoflurane versus total intravenous anaesthesia using propofol and remifentanil. J Int Med Res. 2009;37(6):1760-71. [PubMed ID: 20146874]. https://doi.org/10.1177/147323000903700612.
-
47.
Bulow NM, Barbosa NV, Rocha JB. Opioid consumption in total intravenous anesthesia is reduced with dexmedetomidine: a comparative study with remifentanil in gynecologic videolaparoscopic surgery. J Clin Anesth. 2007;19(4):280-5. [PubMed ID: 17572323]. https://doi.org/10.1016/j.jclinane.2007.01.004.
-
48.
Arcangeli A, D'Alo C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2009;10(8):687-95. [PubMed ID: 19702517]. https://doi.org/10.2174/138945009788982423.
-
49.
Amri P, Nahrini S, Hajian-Tilaki K, Hamidian M, Alipour SF, Hamidi SH, et al. Analgesic Effect and Hemodynamic Changes Due to Dexmedetomidine Versus Fentanyl During Elective Colonoscopy: A Double-Blind Randomized Clinical Trial. Anesth Pain Med. 2018;8(6). e81077. [PubMed ID: 30719412]. [PubMed Central ID: PMC6347670]. https://doi.org/10.5812/aapm.81077.
-
50.
Tilvawala KR, Panchotiya PR. A randomized, comparative study of propofol infusion and sevoflurane as the sole maintenance agent in laparoscopic surgery. Anaesth Pain Intensive Care. 2019:154-8.
-
51.
Akhondzadeh R, Ghomeishi A, Soltani F, Khoshooei A. The Effect of Different Doses of Isoflurane on Hemodynamic Changes and Bleeding in Patients Undergoing Endoscopic Sinus Surgery under General Anesthesia. Anesth Pain Med. 2019;9(1). e57864. [PubMed ID: 30881904]. [PubMed Central ID: PMC6413045]. https://doi.org/10.5812/aapm.57864.