1. Background
Delivery pain is the most unbearable pain for women, associated with psychological effects on the mother after childbirth (1). Various techniques with different drug combinations have been introduced to relieve delivery pain, among which regional analgesia is known as the gold standard technique (2). Spinal anesthesia relieves delivery pain, with its analgesia beginning immediately (3).
Opioids are the most common postoperative pain management drugs (4). Although different drugs have been introduced to manage labor pain, oral and intravenous opioids still play critical roles in painless delivery (5). Adding opioids to neuraxial methods results in more effective analgesia with a longer duration of analgesia and much less motor block. Although opioids are associated with unwanted side effects such as pruritus, neonatal respiratory depression, or fetal heart rate changes, these side effects are dose-dependent. Some recent studies suggest no need to use high doses of neuraxial opioids during delivery analgesia (6). An epidural catheter can provide adequate analgesia when the labor is prolonged; however, single-dose spinal analgesia can be useful in some cases, such as rapid delivery in primiparous and multiparous individuals and in places where the use of an epidural catheter is restricted (7). The proper management of unwanted side effects of intrathecal narcotics may make this method a safe and low-cost method for the delivery of analgesia (8). Regarding intrathecal opioids, fentanyl and sufentanil are safer than meperidine and have fewer effects on neonates (9). A single dose of intrathecal opioids in delivery analgesia has been proven to be more satisfactory than other methods (10). Restricting intrathecal opioids causes short-term analgesia (11). Accordingly, given the analgesia’s ease of implementation, more immediate effects, cost-effectiveness, and sufficient analgesia (12), it can be an acceptable alternative to the epidural in delivery analgesia when the epidural facilities are unavailable (13).
2. Objectives
Given the importance of choosing appropriate drugs and methods for painless delivery and their non-interference with the labor course and neonatal outcomes, we decided to include those mothers undergoing analgesia with intrathecal sufentanil in the Akbarabadi Hospital.
3. Methods
3.1. Design and Setting
This was a single-arm observational cohort study. The study population encompassed 1,055 pregnant women candidates for vaginal delivery with spinal analgesia. The study setting for labor was the Akbarabadi Hospital. Pregnant women lay on the theatre in the supine position with a slight tilt of the uterus to the left side (slight left lateral position) and were monitored non-invasively for blood pressure, heart rate, and Spo2. Spinal anesthesia was done in a sitting position with a spinal needle G26 under sterile conditions after prep and drape at the levels of L3-L4 or L4-L5. Intrathecal sufentanil 0.1 µg/kg was used for all the participants. After injecting the drug, the patient was placed in the supine position, and the body was slightly tilted to the left side, accompanied by blood pressure, heart rate, and fetal heart rate (FHR) monitoring. The participants’ maternal blood pressure and heart rates were recorded after analgesia. During delivery, the mother was monitored for nausea, pruritus, motor block, apnea, urinary retention, or the possibility of an emergency cesarean section. The duration of the analgesia, the duration of the second stage of labor, and the mother's pain were also recorded using the Visual Analogue Scale (VAS). The VAS score was measured during the active contraction phase, and then we calculated the mean VAS. If the VAS was > 3, we planned to use entonox for pain relief. The 1- and 5-minute Apgar scores and arterial blood gas (ABG) of the umbilical cord were also recorded. The Bromage score (score range: 0 - 3) was used to check motor block as the patient’s inability to use the skeletal muscles of the lower extremities (0 = unable to move feet or knees, 1 = just able to move feet, 2 = just able to move knees, and 3 = full flexion of knees and feet). The Bromage score was first measured 10 minutes after spinal anesthesia and then every other 10 minutes up to 30 minutes. The sedation level was also evaluated using the RSS [ramsay sedation scale] (1) patient anxious, agitated, or restless; (2) patient cooperative, oriented, tranquil, and alert; (3) patient responds to commands; (4) asleep, but with brisk response to a light glabellar tap or loud auditory stimulus; (5) asleep, sluggish response to a light glabellar tap or loud auditory stimulus; (6) asleep, no response). The RSS values were recorded every other 60 minutes during labor. The Bromage score (score range: 0 - 3) was used to check motor block as a patient’s inability to use the skeletal muscles of the lower extremities (0 = unable to move feet or knees, 1 = just able to move feet only, 2 = just able to move knees, and 3 = full flexion of knees and feet). The Glasgow Coma Scale/Score (GCS) was used to check the consciousness level. Hypotension was defined as a drop in systolic blood pressure by < 90 mmHg or < 20% of baseline, which was treated by ephedrine 10 mg bolus. Bradycardia was defined as a heart rate drop of < 50 beats per minute or < 20% of baseline, which was treated with atropine 0.01 mg/kg. The duration of analgesia and the duration of the second stage of labor were recorded in minutes. Fetal distress was assessed using continuous non-stress test (NST) monitoring.
3.2. Eligibility Criteria
Inclusion criteria were as follows: (1) 18 - 45 years old, (2) candidates for vaginal delivery using the spinal method, (3) American Society of Anesthesiologists (ASA) I and II, (4) term pregnancy, (5) singleton, and (6) vertex presentation. The following exclusion criteria were also considered: (1) history of sufentanil sensitivity, (2) contraindications for spinal, (3) prematurity, (4) fetal abnormality, and (5) prescription of previous systemic opioids, and (6) unwillingness to participate.
3.3. Ethical Issues
The research followed the principles of the Declaration of Helsinki. The Ethics Committee of the Iran University of Medical Sciences approved this study. The institutional ethics committee affiliated with the Iran University of Medical Sciences approved all study protocols (code: IR.IUMS.REC.1399.520).
3.4. Statistical Analysis
The collected data were analyzed with SPSS software version 21. All quantitative variables had normal distribution, and descriptive statistics (mean, standard deviation, frequency, and percentage) were used. In the present study, the significance level was < 0.05.
4. Results
This study included 1,055 pregnant women with a gestational age of 38.61 ± 9.81 months. Mean gravidity was 2.78 ± 1.56, dilation was 4.12 ± 1.71 cm, and effacement was 60.13 ± 8.83 % (Table 1).
| Variables | Min. | Max. | Mean ± Standard Deviation |
|---|---|---|---|
| Gravidity | 1 | 4 | 2.78 ± 1.56 |
| Gestational age | 37.1 | 40 | 39.61 ± 2.81 |
| Dilation, cm | 3 | 6 | 4.12 ± 1.71 |
| Effacement, % | 50 | 70 | 60.13 ± 8.83 |
The fetal station can be described anywhere the baby is located on a scale of -3 to +3, with 0 at the level of the ischial spine. In this regard, the most common station was -3 for 723 women, followed by -2 for 229 women. It should be noted that the vacuum was used just for a patient with an effacement of 60%, a gravidity of 2, a dilation of 6, and a station of -2.
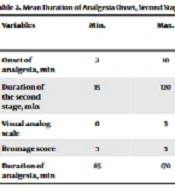
In this study, 52 pregnant women underwent cesarean section. We assessed the causes of cesarean section in four categories: Fetal distress, failure of progress, cord prolapse, and fetal tachycardia. The most common cause was heart failure (57.7%), and the failure of progress, cord prolapse, and fetal tachycardia accounted for 36.5%, 3.8%, and 1.9% of the cases. The mean period for the onset of painlessness was 5.93 ± 2.87 minutes, and the mean visual analog scale was 1.08 ± 0.16. The mean duration of the second stage was 31.29 ± 11.82 minutes, and the mean duration of analgesia was 125 ± 27.32 (Table 2).
| Variables | Min. | Max. | Mean ± Standard Deviation |
|---|---|---|---|
| Onset of analgesia, min | 2 | 10 | 5.93 ± 2.87 |
| Duration of the second stage, min | 15 | 120 | 31.29 ± 11.82 |
| Visual analog scale | 0 | 3 | 1.08 ± 0.16 |
| Bromage score | 3 | 3 | 3 |
| Duration of analgesia, min | 85 | 170 | 125 ± 27.32 |
Table 3 presents the neonates’ clinical characteristics. As presented in this table, mean Apgar is 9.0 ± 0.47, birthweight is 2917.39 ± 449.90 gr, PH is 7.31 ± 2.03, HCO3 is 22.67 ± 3.08 mEq/liter, and PaCo2 is 43.36 ± 7.06 mmHg, and base excess if -1.41 ± 2.32. It should be noted that all patients had no motion blockage; hence, their Bromage score was 3.
| Variables | Min. | Max. | Mean ± Standard Deviation |
|---|---|---|---|
| The interval between "fetal heart rate drop" and spinal anesthesia, min | 1 | 160 | 78.02 ± 43.41 |
| Apgar | 8 | 10 | 9.06 ± 0.47 |
| Weight, gr | 2560 | 3180 | 2917.39 ± 449.90 |
| PH | 7.28 | 7.45 | 7.31 ± 2.03 |
| HCO3, mEq/liter | 22 | 26 | 22.67 ± 3.08 |
| Base excess | -4 | 3 | -1.41 ± 2.32 |
| PaCo2, mmHg | 35 | 45 | 43.36 ± 7.06 |
5. Discussion
This study investigated the effect of intrathecal sufentanil for painless delivery on labor progress and neonatal outcomes in 1,055 pregnant women with a gestational age of 38.61 ± 9.81 months. In summary, the findings revealed that mean gravidity, dilation, and effacement were 2.78 ± 1.56, 4.12 ± 1.71 cm, and60.13 ± 8.83 %, respectively. The most common station was -3 for 723 women, followed by -2 for 229 women. We assessed the cause of cesarean section performed on 52 pregnant women in four categories: Fetal distress, failure of progress, cord prolapse, and fetal tachycardia. The most common cause of cesarean section was fetal distress (57.7%). The mean period of the onset of analgesia was 5.93 ± 2.87 minutes, and the mean visual analog scale was 1.08 ± 0.16. Mean Apgar was 9.0 ± 0.47, normal PH in the fetus was reported to range from 7.25 to 7.45 (14), and 7.31 ± 2.03 in the present study, indicating that intrathecal sufentanil made no acidosis. As mentioned, intrathecal sufentanil resulted in patients’ high satisfaction aroused by the low score of VAS for painless delivery in the short term (mean: 5.93 minutes). These results documented the safety of this pharmaceutical agent in pregnant women. Imani et al. found that spinal analgesia is a simple procedure providing a lower pain score than epidural analgesia (15). In Wang et al.’s meta-analysis study, the doses of fentanyl and sufentanil usually used in spinal and epidural techniques were safe for neonatal outcomes, and there was no difference between the intervention and control groups regarding Apgar and umbilical cord pH (1). Their findings were consistent with the findings of the present study. In AbdElBarr et al.’s study, a group received 3.75 mg of bupivacaine, 25 mg of fentanyl by spinal injection, 4 mL of bupivacaine, and 50 mg of fentanyl were injected epidurally in another group. In this study, in the spinal group, the onset of the sensory block was faster, and the duration of the sensory block was longer. VAS and the incidence of hypotension were lower in the spinal group. The incidence of motor block, sedation, and nausea were equal in the two groups. The incidence of pruritus was higher in the spinal group (13). Regarding complications, the patients just developed itching (n = 78), and other complications, including hypotension, bradycardia, apnea, and decreased level of consciousness, did not appear, indicating the safety of the sufentanil use as an intrathecal agent. In line with our findings, AbdElBarr et al. (13) showed the safety of fentanyl; however, nausea and vomiting were not observed. Sufentanil has lower nausea compared to pethidine, as Salarian et al.’s study on 600 pregnant women documented this finding. In this study, a group received 0.4 mg/kg intrathecal pethidine, and another group received 0.1 µg/kg intrathecal sufentanil. The group receiving pethidine had a higher rate of nausea than the other group, and the group receiving sufentanil had more frequent itching than the other group. Significant analgesia was observed for labor in both groups (3). In the present study, 78 patients developed itching.
To continue analgesia in patients receiving intrathecal sufentanil, the repeated administration of enough sufentanil from a specific catheter is required. Minty et al. conducted a meta-analysis and reported that intrathecal drugs safely reduced pain in pregnant women during labor. The mothers were more satisfied with this method than the other methods. The limitation of intrathecal drugs is their short duration of analgesia; however, it may be the best method where the other techniques are not available (7).
In the present study, intrathecal sufentanil 0.1 µg/kg was used for all women. Wang et al. claimed intrathecal sufentanil 0.5 µg induced rapid analgesia (16). Vaananen et al. reported that considering the reduction of VAS score at 20 minutes, sufentanil provided better results compared to fentanyl (17). Similar to our findings, some evidence suggests that sufentanil should be used for analgesia during labor compared with fentanyl. Therefor it is more effective in keeping the duration of spinal analgesia with safer results regarding neonate outcomes (18). In our study, the mean period for the onset of analgesia was 5.93 minutes, and the mean pain score was 1.08, indicating the suitable and timely painless delivery of pregnant women.
A large number of studies, in addition to our study showed promising results in using intrathecal sufentanil 0.1 µg/kg regarding the onset of analgesia, high Apgar, normal ABG, and not delayed second stage time. Furthermore, the distance between spinal and fetal distress (80.59 minutes) indicated that spinal anesthesia did not cause fetal distress.
5.1. Conclusions
Intrathecal sufentanil resulted in patients’ high pain due to the low score of VAS for painless delivery in a short period (mean: 5.93). Following the use of intrathecal sufentanil, normal Apgar (mean: 9.06) and normal PH (mean: 7.31) indicated the safety of this pharmaceutical agent for pregnant women. In randomized clinical trials, it is recommended to compare intrathecal sufentanil with other opioids.
5.2. Limitations of the Study
This study was conducted in the Aliakbarabadi Hospital. Further studies with larger sample sizes are recommended to evaluate the effect of the underlying causes of cesarean section. This study did not aim to analyze patients in different subgroups. Accordingly, further studies with control groups are recommended. The present study was a single-arm cohort observational study with no control group. Furthermore, the case group and controls can be compared to evaluate the exact effect of spinal anesthesia on birthweight.