Abstract
Background:
Despite all of the benefits provided by laparoscopic cholecystectomy, such as rapid recovery and shorter hospital stay for patients, the incidence of postoperative nausea and vomiting (PONV) and postoperative pain (POP) still remains high.Objectives:
This study was designed to compare the effects of intraperitoneal (IP) and intravenous (IV) dexamethasone on the reduction of PONV and POP.Methods:
This prospective, randomized, double-blind clinical trial was conducted on a study population of 86 adult patients who were scheduled for laparoscopic cholecystectomy with the American Society of Anesthesiologists class I-II. The patients were randomized into three groups, namely IP dexamethasone (n = 29), IV dexamethasone (n = 29), and control (n = 28) groups. The patients were followed for clinical outcomes, including PONV, POP, and consumption of antiemetics, and their hemodynamic status during the first 24 hours after the surgery.Results:
In the first 24 hours after the operation, no significant differences were observed in nausea (P = 0.41) and vomiting (P = 0.38) between the IP and IV dexamethasone groups. However, there was a lower severity of nausea in the IP group (P = 0.001). Additionally, the visual analog scale score representing POP was significantly reduced in the IP group (P = 0.02). No significant differences in the hemodynamic status were observed after the operation between all the three groups.Conclusions:
The administration of 8 mg IP dexamethasone was associated with significantly reduced pain and severity of nausea, but not PONV, after laparoscopic cholecystectomy.Keywords
Cholecystectomy Dexamethasone Intraperitoneal Laparoscopic Postoperative Nausea and Vomiting Pain
1. Background
Minimally invasive techniques for surgery, such as laparoscopic cholecystectomy, provide various benefits to patients, including faster recovery, shorter hospital stay, and a rapid return to normal activities (1, 2). However, a high incidence of postoperative nausea and vomiting (PONV) and postoperative pain (POP) remains a remarkable problem that can negatively affect patient satisfaction and might consequently cause adverse side effects (3-6). The PONV is one of the most common complications after general anesthesia (7-9), with an overall incidence of up to 30% in all surgeries and about 52 - 80% in patients undergoing laparoscopic cholecystectomy (10). Several factors have been identified that increase the risk for PONV, such as female gender, a history of PONV, history of motion sickness, nonsmoking status, use of opioids, and long duration of surgery (10-12). Since 1981, dexamethasone has been recognized as a potent antiemetic for the treatment of nausea in patients who received chemotherapy as a therapy for cancer (3). Dexamethasone, well known for its antiemetic effects, is a glucocorticoid. The exact mechanism of action of dexamethasone remains unclear. An explanation might be the effects of dexamethasone on the vomiting center at the medulla oblongata and central nervous system, such as blood-brain-barrier permeability alterations to some blood proteins, changes in neurotransmitters activity, such as serotonin and dopamine, or suppression of prostaglandins production (10, 13).
Dexamethasone use has been reported for its antiemetic effect in various situations, such as after chemotherapy, since 1981 and for pediatrics surgeries, thyroidectomy, and major gynecological surgeries since 1999, with equal or more antiemetic effects, compared to other common agents, such as serotonin (5HT-3) receptor antagonists (8). In most of the previous studies, dexamethasone was used in a single dose with different amounts (e.g., 4, 6, and 8 mg in different studies), in different combinations, via intravenous (IV) route of injection to prevent PONV, while having in mind some side effects, such as the risk of postoperative infections and transient hyperglycemia (10-15). Therefore, based on the evidence, a single-dose IV dexamethasone injection was considered an effective and common antiemetic to prevent PONV (15).
In recent years, some researchers have tried to find a more effective way to reduce PONV, pain, and the frequent consumption of analgesic and antiemetic medications during the first 24 hours after operations to relieve discomfort in patients. A single dose of intraperitoneal (IP) dexamethasone injection in gynecological surgeries has been associated with lower pain and PONV. Researchers claimed that IP dexamethasone injection is also associated with lower side effects (e.g., dizziness and headache) than IV injection (7, 10, 16-19). Therefore, the current study was performed to compare the effect of IP versus IV dexamethasone on PONV and POP after laparoscopic cholecystectomy.
2. Objectives
To the best of our knowledge, no previous studies in the literature have compared the effect of IP versus IV dexamethasone on the incidence of PONV and POP after laparoscopic cholecystectomy. The present study hypothesized that the direct injection of dexamethasone in the IP cavity would reduce the incidence of PONV and POP as primary outcomes of the study after laparoscopic cholecystectomy.
3. Methods
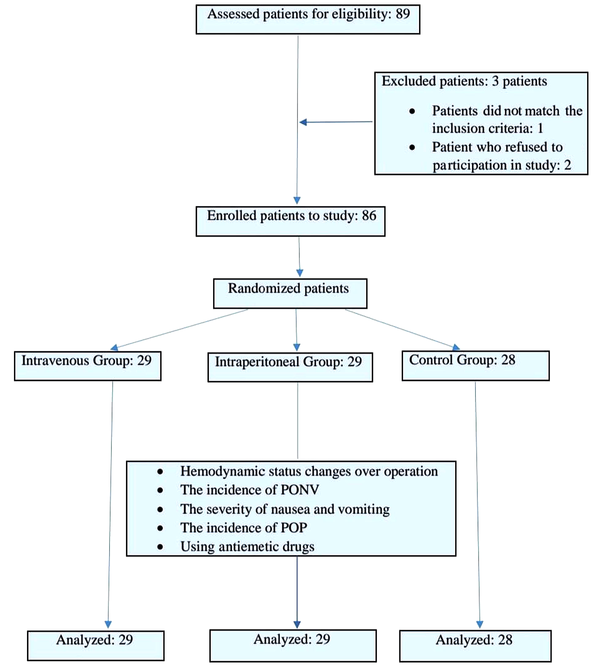
This prospective, randomized, double-blind clinical trial was conducted in the Amin and Al-Zahra hospitals, Isfahan University of Medical Sciences, Isfahan, Iran, within 2020-2021 to compare the effects of IP and IV dexamethasone injection on PONV and POP after laparoscopic cholecystectomy. A primary population of about 89 patients was considered for entering the study. After the exclusion of 3 individuals, all remaining 86 patients were equally randomized into two groups of 29 patients and one group of 28 who were candidates for laparoscopic cholecystectomy (Figure 1).
Study flowchart
The following formula was used to obtain the sample size:
For blinding to the selection method, all patients received 2 mg of midazolam before entering the operating room. They received dexamethasone and normal saline via IP and IV routes after ending the operation, and the results were compared. Dexamethasone and saline were directly injected (and not sprayed) in the peritoneum of the gallbladders bed just before putting out the trochar of laparoscope at the end of the operation. Dexamethasone was manufactured by Iran Hormone Pharmaceutical Company. The primary evaluations, such as noninvasive blood pressure monitoring, pulse rate, heart rate, respiratory rate, electrocardiographic studies, oxygen saturation, and capnography, were performed for all patients after they entered the operation room.
The anesthesia induction method was receiving 1 mg/kg of IV midazolam, 2 μg/kg of IV fentanyl, 5 mg/kg of thiopental sodium, and 0.15 mg/kg of IV cisatracurium in all patients. The subjects were randomly assigned into three groups; group A (IV) received 8 mg of IV dexamethasone and 2 cc of IP normal saline as the placebo (after last clamp insertion and before laparoscopic trocar withdrawal); group B (IP) received 8 mg of IP dexamethasone (in the subhepatic peritoneum) and 2 cc of IV normal saline as the placebo; group C (control) received 2 cc of IP normal saline and 2 cc of IV normal saline. All the operations were performed under the same protocol, with the same surgeon and surgical technique.
All the patients were prohibited from taking medications 24 hours before the surgery. After ending the surgery, the patients were transferred to the recovery ward. After their awakening, their length of stay in recovery was checked using the Modified Aldrete Score system; then, they were assessed for the objectives of the study, including complaining of PONV, the severity of nausea, the severity of vomiting, and pain. After complete recovery and transfer to the Surgery Ward, the patients continued to be monitored for the required objectives, and in case of complaining about PONV, they received an antiemetic (i.e., metoclopramide in the current study) for 24 hours.
At the end of the study period, the patients were assessed for satisfaction with their surgery experience using the Likert system. For the assessment of pain and severity of nausea and vomiting, the visual analog scale (VAS) was used (from 0 for no pain, nausea, and vomiting to 10 for worst possible pain and most severe nausea and vomiting), in which the patients were asked to report the severity of pain, nausea, and vomiting within 2, 4, 8, 12, and 24 hours after laparoscopy. After the operation, a blinded (non informed) observer asked the patients about nausea and vomiting and recorded the results into a checklist. The patients, who complained of PONV, received 10 mg of IV metoclopramide and, in the second line, 4 mg of ondansetron. In addition, to rescue from pain, the patients received a diclofenac suppository and, in the second line, 3 mg of IV ketorolac.
The inclusion criteria were as follows: Patients who were scheduled for laparoscopic cholecystectomy with:
(1) More than 18 years of age
(2) The American Society of Anesthesiologists class I-II
(3) Consent for participation in the study
The exclusion criteria were as follows:
(1) Patients with sensitivity to dexamethasone
(2) Patients who refused to continue participation in the study
(3) Patients with a change in the treatment method
The non-inclusion criteria were as follows:
(1) Patients with pregnancy
(2) Previous allergic reaction to anesthesiology drugs
(3) Previous allergic reaction to dexamethasone
(4) Patients who were under anticoagulant medicines
(5) Those who received systemic corticosteroids in the 24 hours before surgery
All eligible patients included in the study were asked to sign a written informed consent before participation after receiving an explanation about the study by researchers. All the patients were informed about the study goals, content, and interventions before entering the study. Those who agreed to participate in the study signed informed consent forms. The ethical issues and the protocol were reviewed and approved by the Ethical Committee of Isfahan University of Medical Sciences. The running protocol was devised by the researchers of this study (IR.MUI.MED.REC.1399.350).
One-way analysis of variance (to compare the differences of means), the Fisher’s exact test (to examine the relationships of qualitative variables), repeated measures test (to compare the quantitative variables between the groups), chi-square test (to determine differences of expected frequencies), and generalized linear model (to describe the relationships of predictors and outcomes of intervention) were used for data analysis. All collected data were analyzed using SPSS software (version 23). P-values less than 0.05 were considered statistically significant.
4. Results
A total of 86 patients were enrolled to participate in the study. The administration of metoclopramide to relieve in patients who experienced PONV was significantly higher in the IV group than in the IP group (P = 0.001). No statistically significant differences between the three groups were observed in terms of age (P = 0.38), operation duration (P = 0.14), anesthesia method (P = 0.08), and recovery duration (P = 0.42). Nevertheless, weight (P = 0.007) and consequently body mass index (P < 0.001) were significantly higher in the IV administration group. The effect of weight in all future analyses and the effect of metoclopramide in the analyses that pertained to recovery were adjusted to ensure the accuracy of the results. The mean duration of surgery was not significantly different between the three groups (P = 0.14). Table 1 shows the aforementioned results.
| Variable | Group | |||
|---|---|---|---|---|
| Control | Intravenous | Intraperitoneal | P-Value b | |
| Age (y) | 40.1 ± 8.6 | 41.8 ± 8.4 | 42.1 ± 14.4 | 0.38 |
| Weight (kg) | 88.5 ± 9.4 | 97.7 ± 19.8 | 86.9 ± 8.5 | 0.007 |
| Body mass index (kg/m2) | 31.6 ± 4.0 | 36.5 ± 6.2 | 31.9 ± 3.2 | < 0.001 |
| Surgery duration (min) | 38.1 ± 6.7 | 42.9 ± 11.3 | 40.2 ± 8.3 | 0.14 |
| Length of stay in recovery (min) | 61.9 ± 11.9 | 60.8 ± 15.1 | 58.1 ± 6.0 | 0.42 |
| Anesthesia prolongation (min) | 100.8 ± 14.1 | 106.8 ± 19.1 | 98.1 ± 7.4 | 0.08 |
| Metoclopramide dose (mg) | 9.6 ± 3.5 | 12.0 ± 0.0 | 4.0 ± 3.9 | 0.001 |
Overall, 65 (75.9%) and 21 (24.1%) patients were female and male, respectively. The underlying disease of patients was acute cholecystitis. Satisfaction with the surgery experience was reported as very good, good, moderate, bad, and very bad by 26 (31%), 32 (38.1%), 16 (19%), 9 (10.7%), and 1 (1.2%) patients (P < 0.001), respectively. Table 2 shows the details of different study groups.
| Variable | Group | P-Value b | |||
|---|---|---|---|---|---|
| Control | Intravenous | Intraperitoneal | Total | ||
| Gender | 0.26 | ||||
| Male | 6 6.9 (6) | 11.5 (10) | 5.7 (5) | 24.1 (21) | |
| Female | 26.4 (22) | 22.8 (19) | 27.6 (24) | 75.9 (66) | |
| Underlying disease | 0.40 | ||||
| Cholecystitis | 40.3 (27) | 23.9 (16) | 34.3 (22) | 98.5 (66) | |
| Very bad | 1.2 (1) | 0 (0) | 0 (0) | 1.2 (1) | |
| Bad | 6 (5) | 4.8 (4) | 0 (0) | 10.7 (9) | |
| Satisfaction with surgery experience | < 0.001 | ||||
| Moderate | 13.1 (11) | 3.6 (3) | 2.4 (2) | 19 (16) | |
| Good | 13.1 (11) | 10.7 (9) | 14.3 (12) | 38.1 (32) | |
| Very good | 1.2 (1) | 13.1 (11) | 16.7 (14) | 31 (26) | |
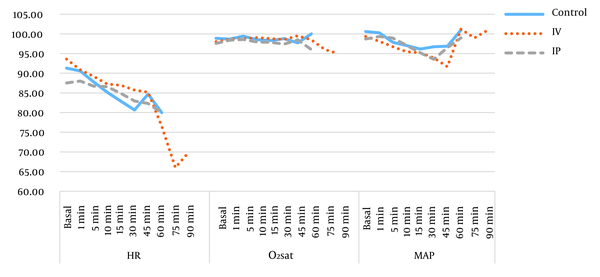
There were no significant differences in the heart rate (P = 0.45), oxygen saturation (P = 0.23), mean arterial pressure (P = 0.12), and surgery duration between the three groups of study, as shown in Figure 2.
Heart rate, oxygen saturation, and mean arterial pressure during operation.
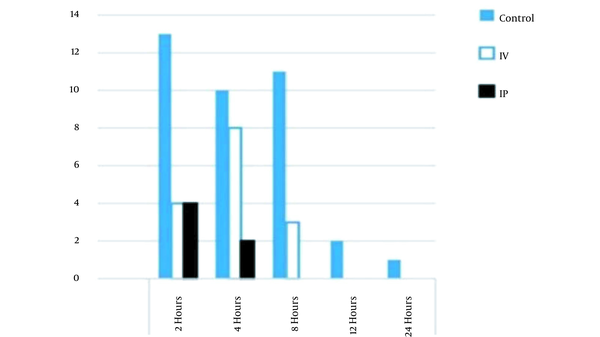
The results showed that the incidence of nausea was reduced in the IP group in comparison to that of the control group (but not in the IV group) in the first 24 hours after the surgery; however, unlike the IV group, none of the IP group patients experienced PONV 8 hours after the operation, as shown in Figures 3 and 4.
Comparison of nausea incidence between groups in first 24 hours after operation; notes: IP and IV are abbreviations for intraperitoneal and intravenous, respectively.
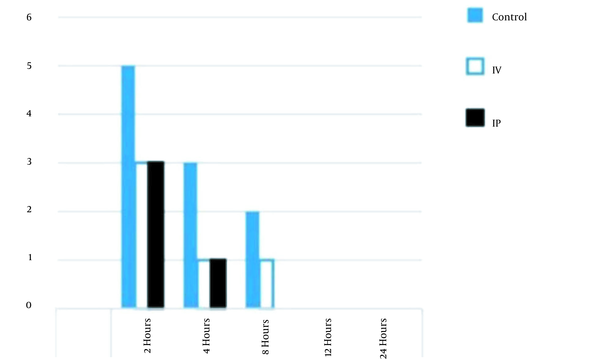
Comparison of vomiting incidence between groups in first 24 hours after operation; notes: IP and IV are abbreviations for intraperitoneal and intravenous, respectively.
There were also no significant differences in nausea (P = 0.41) and vomiting (P = 0.38) between the three groups, as shown in Table 3.
| Variable and Time | Group | P-Value b | ||
|---|---|---|---|---|
| Intraperitoneal | Intraperitoneal | Intraperitoneal | ||
| Nausea | 0.38 | |||
| 2 h | 15.1 (13) | 4.8 (4) | 5.8 (5) | |
| 4 h | 11.6 (10) | 9.3 (8) | 3.5 (3) | |
| 8 h | 12.8 (11) | 3.5 (3) | 0 (0) | |
| 12 h | 2.3 (2) | 0 (0) | 0 (0) | |
| 24 h | 1.2 (1) | 0 (0) | 0 (0) | |
| Vomiting | 0.41 | |||
| 2 h | 5.8 (5) | 3.5 (3) | 3.5 (3) | |
| 4 h | 3.5 (3) | 1.2 (1) | 1.2 (1) | |
| 8 h | 2.3 (2) | 1.2 (1) | 0 (0) | |
| 12 h | 0 (0) | 0 (0) | 0 (0) | |
| 24 h | 0 (0) | 0 (0) | 0 (0) | |
Furthermore, the incidence of postoperative vomiting was reduced in comparison to that of the control and IV groups. In addition, none of the IP group patients experienced postoperative vomiting 8 hours after the operation. The incidence of PONV was equal between the IP and IV groups 2 hours after the operation, as shown in Figure 4. The results showed a significant reduction in the severity of nausea (P = 0.001) and pain levels (P = 0.02) in the IP group patients, compared to those of the control and IV groups, as shown in Table 4.
| Variable and Time | Group | P-Value | ||
|---|---|---|---|---|
| Control | Intravenous | Intraperitoneal | ||
| Severity of nausea | 0.001 | |||
| 2 h | 2.00 ± 2.3 | 1.76 ± 0.5 | 1.42 ± 0.5 | |
| 4 h | 1.10 ± 1.9 | 1.91 ± 0.3 | 0.65 ± 0.3 | |
| 8 h | 1.38 ± 1.8 | 1.41 ± 0.2 | 0.0 | |
| 12 h | 0.34 ± 1.0 | 0.0 | 0.0 | |
| 24 h | 0.14 ± 0.7 | 0.0 | 0.0 | |
| Severity of vomiting | 0.26 | |||
| 2 h | 1.35 ± 0.5 | 1.35 ± 0.4 | 1.404 ± 0.4 | |
| 4 h | 1.26 ± 0.3 | 1.265 ± 0.1 | 0.743 ± 0.1 | |
| 8 h | 0.66 ± 0.1 | 0.658 ± 0.1 | 0.73 ± 0.1 | |
| 12 h | 0.0 | 0.0 | 0.0 | |
| 24 h | 0.0 | 0.0 | 0.0 | |
| Pain | 0.02 | |||
| 2 h | 2.086 ± 1.7 | 2.19 ± 1.4 | 1.27 ± 0.5 | |
| 4 h | 1.34 ± 0.9 | 1.67 ± 0.9 | 0.77 ± 0.2 | |
| 8 h | 1.24 ± 0.5 | 1.30 ± 0.5 | 0.55 ± 0.1 | |
| 12 h | 0.73 ± 0.2 | 0.56 ± 0.1 | 0.0 | |
| 24 h | 0.0 | 0.0 | 0.0 | |
5. Discussion
The current study was performed to compare the effects of IP and IV dexamethasone on the prevention of PONV and POP in patients who underwent laparoscopic cholecystectomy. The results obtained in this study were also used to investigate the effect of IP dexamethasone on the hemodynamic status of patients. In a meta-analysis study, the authors demonstrated that granisetron, a commonly used antiemetic drug, could prevent PONV more effectively combined with dexamethasone (20).
Moreover, numerous randomized controlled trials (RCTs) investigated the combination of dexamethasone and other antiemetics in different doses to evaluate the prophylactic effects on PONV in laparoscopic cholecystectomy. However, the population in such trials was usually limited, which meant that the results were probably uncertain. A recent meta-analysis suggested that 8 mg of IV dexamethasone acts as the optimal effective dose for the prevention of PONV, especially when combined with ondansetron. The studies that used the aforementioned dose reported a lower incidence of PONV and a lower rate of antiemetic drugs consumption (15).
Some studies, including a study performed by Elhakim et al., suggested that the routine preoperative administration of IV dexamethasone, alone or in combination with other oral antiemetic medications, such as ondansetron or metoclopramide, reduced the risk of PONV (21). Feo et al. suggested that the preoperative administration of dexamethasone, alone and without other antiemetic medications, could prevent PONV after laparoscopic cholecystectomy (22). All the aforementioned studies demonstrated that dexamethasone could act as a potent antiemetic to prevent PONV when used intraoperatively. The current study demonstrated that the IP administration of dexamethasone could significantly reduce the severity of nausea after the operation; however, this effect was not shown to act on the severity of vomiting and the incidence of PONV. There are discrepancies between the results, which could be due to different doses, routes of administration, or surgical techniques. In addition, the risk factors of nausea and vomiting were not considered in all studies and could be responsible for the differences in the results.
The POP is another common side effect of laparoscopic operations and usually occurs due to incision wounds, visceral pain, and diaphragm irritation which causes pneumoperitoneum and shoulder pain (22). Consequently, uncontrolled pain can make patients dissatisfied with the quality of surgery and improve the risk of morbidity and mortality (16). In recent years, investigations on analgesics have made remarkable advantages in postlaparoscopic pain management (8). For example, one of such recent randomized controlled studies by Gayam et al. suggested that combining IV dexamethasone with other analgesics, such as IP bupivacaine, could be significantly effective in the reduction of PONV and POP (8).
Previous studies postulated that IP dexamethasone could act as a potent agent to relieve shoulder pain in females who underwent laparoscopic gynecological operations. In one such method, Asgari et al. demonstrated shoulder pain to be a significant side effect after gynecological laparoscopy, which occurred due to carbon dioxide pneumoperitoneum (usually occurring due to diaphragm injury during laparoscopy) (7). The results showed that the pain was significantly lower in severity in patients who received a single dose of 16 mg of IP dexamethasone, compared to that of the placebo group of patients. The aforementioned study also reported that the postoperative administration of analgesic drugs, such as opioids and narcotics, was lower in patients with IP dexamethasone than in the placebo group (7).
Moreover, Hosseini Valami et al. compared the effects of bupivacaine, dexamethasone, and morphine to the placebo (saline) in the IP route of administration on pain after a caesarian section in 144 pregnant women (16). The aforementioned study reported that patients who received 16 mg (diluted to 30 cc) of IP dexamethasone and 30 cc of bupivacaine (25%) experienced lower pain and had a lower VAS score, compared to patients who received 5 mg (diluted to 30 cc) of IP morphine (16).
Some studies compared the effects of different doses of intraoperative dexamethasone with or without other drugs on POP. Sultan et al. reported that the preoperative administration of 0.1 mg/kg single-dose IV dexamethasone could enhance the quality of patient recovery and improve pain control after laparoscopic cholecystectomy, compared to lignocaine (23). Additionally, Elsakka et al. studied the effect of different corticosteroids on POP and proved that the administration of IP dexamethasone and hydrocortisone could reduce abdominal pain and shoulder pain in patients and consequently reduce the need for the administration of analgesics after laparoscopic cholecystectomy, without causing any significant side effect to patients (23, 24).
A systematic review in collaboration with the international Procedure Specific Postoperative Pain Management (PROSPECT) that aimed to establish proper protocols for the effective management of postoperative laparoscopic cholecystectomy pain reviewed clinical trials within 2006 (after the previous version) to 2017. The results of the aforementioned study recommended preoperative dexamethasone as an effective medication to reduce POP (up to 48 hours after the operation) and PONV. The aforementioned study recommended a combination of preoperative dexamethasone with metoclopramide, ondansetron, and rofecoxib to reduce the levels of the most severe pain that was experienced by patients (25). However, some clinical trials reported contrasting results. For instance, Mohtadi et al., in a randomized, double-blind study on 122 patients, reported that no significant difference was witnessed in POP between dexamethasone group patients (who received up to 8 mg of IV dexamethasone) and control group patients (who received 2 mL of normal saline as the placebo) (26). Ali et al., in a study on 75 patients, reported that pain scores were significantly lower in patients receiving ondansetron-bupivacaine than patients receiving dexamethasone-bupivacaine and the control group (receiving saline-bupivacaine) (27).
In addition, Zahra et al. reported that the IP administration of bupivacaine-magnesium sulfate was a more effective combination in the reduction of POP and analgesic consumption, compared to bupivacaine-dexamethasone, in a population of about 60 patients undergoing laparoscopic cholecystectomy (28). The researchers also suggested that this could be due to the prolonged anesthesia duration due to the use of the bupivacaine-magnesium sulfate combination and nalbuphine consumption after operation (28). As previously mentioned, studies demonstrated the dexamethasone effect as a potent drug to reduce POP when used intraoperatively. However, there are discrepancies between the results, which could be due to different doses of used dexamethasone, surgeon skills, or even different combinations of dexamethasone and other drugs. The current study showed that the administration of 8 mg IP dexamethasone intraoperatively was associated with a significant POP reduction in comparison to 8 mg IV dexamethasone and the placebo. No drug combination was used in the current study, and dexamethasone was used only with the placebo.
Furthermore, the patients’ hemodynamic status was another outcome that was evaluated in the current study. Other researchers also studied this issue. For example, a study performed by Rajnikant et al. reported no significant differences in mean arterial pressure, heart rate, and blood oxygen saturation during surgery between the two intervention groups (i.e., dexamethasone-palonosetron and dexamethasone-ondansetron), compared to the control (saline) group (29). Similarly, in the current study, there was no significant difference in the hemodynamic status of patients during the surgery between the IV, IP, and control groups.
Alkaissi et al. compared the antiemetic effect of dexamethasone with and without metoclopramide on the incidence and severity of nausea and pain in about 120 patients (30). The authors reported that a combination of dexamethasone-metoclopramide, compared to metoclopramide or dexamethasone alone, significantly reduced the severity of nausea; however, it did not affect the incidence of nausea. Alkaissi et al. also demonstrated the effect of this combination on the incidence and severity of pain after laparoscopic operations (30).
Another double-blind RCT study performed by Ismail et al. on 80 participant females undergoing gynecological laparoscopies compared the effect of IP and IV dexamethasone (and not a placebo) on PONV and POP (10). They reported that IP dexamethasone could reduce POP and the need for meperidine 24 hours after gynecologic cholecystectomy more effectively, compared to IV dexamethasone. Ismail et al. also demonstrated IP dexamethasone to reduce the incidence of PONV in the first 24 hours after gynecologic laparoscopies (experience of nausea and vomiting in 16 and 5 patients in the IV and IP groups, respectively). However, this effect was not reported similarly regarding the severity of nausea in patients (10).
Furthermore, in a recent study, Nouri et al. evaluated the effects of IP dexamethasone (and not IV) on PONV and shoulder pain in 130 patients undergoing gynecologic laparoscopy (31). The results showed that about 40% of the IP dexamethasone group experienced PONV, compared to that of the control (placebo) group. Nouri et al. also reported that the mean VAS score in the IP dexamethasone group was lower than the placebo group during the first 24 hours after the operation (31). The researchers suggested considering IP dexamethasone for routine administration in gynecologic laparoscopic operations (31). Nonetheless, the current study demonstrated that IP dexamethasone could significantly reduce the severity of nausea (and not the incidence of PONV and severity of vomiting) after the operation. On the other hand, the patients who received IP dexamethasone during laparoscopic cholecystectomy experienced lower pain than the IV and control groups.
The current study concluded that the administration of 8 mg IP dexamethasone intraoperatively in laparoscopic cholecystectomy was associated with reduced PONV during the first 24 hours after the operation. However, this antiemetic feature of IP dexamethasone was not reported as a significant effect. Nevertheless, 8 mg dexamethasone administered intraoperatively significantly reduced POP and the subsequent use of analgesics during the 24 hours after laparoscopic cholecystectomy, compared to the results of the IV and control groups.
Different from previous studies that have been performed over the past years, the current study not only investigated the effect of the IP dexamethasone administration on PONV, compared to the control group, but also compared it to that of IV dexamethasone administration. In addition, the present study considered the effect of dexamethasone on both PONV and POP and not just one complication. However, the current study had some limitations, including the limited study population and no evaluation of the side effects of dexamethasone in each group. It is suggested to carry out further studies on larger populations, different doses, and different timing intervals to compare the effects of IP and IV dexamethasone administration during surgery to achieve enough evidence to recommend the routine administration of IP dexamethasone for preventing the complications of laparoscopic cholecystectomy (e.g., PONV and POP) and consequently increasing patient satisfaction. Furthermore, there is a need for a systematic review and meta-analysis to collect all the results of different studies.
References
-
1.
Suksompong S, von Bormann S, von Bormann B. Regional Catheters for Postoperative Pain Control: Review and Observational Data. Anesth Pain Med. 2020;10(1). e99745. [PubMed ID: 32337170]. [PubMed Central ID: PMC7158241]. https://doi.org/10.5812/aapm.99745.
-
2.
Edinoff AN, Girma B, Trettin KA, Horton CC, Kaye AJ, Cornett EM, et al. Novel Regional Nerve Blocks in Clinical Practice: Evolving Techniques for Pain Management. Anesth Pain Med. 2021;11(4). e118278. [PubMed ID: 34692446]. [PubMed Central ID: PMC8520672]. https://doi.org/10.5812/aapm.118278.
-
3.
Gupta P, Khanna J, Mitramustafi AK, Bhartia VK. Role of pre-operative dexamethasone as prophylaxis for postoperative nausea and vomiting in laparoscopic surgery. J Minim Access Surg. 2006;2(1):12-5. [PubMed ID: 21170221]. [PubMed Central ID: PMC2997215]. https://doi.org/10.4103/0972-9941.25671.
-
4.
Davari-Tanha F, Samimi S, Khalaj Z, Bastanhagh E. Comparison of Intraperitoneal Normal Saline Infusion with Pulmonary Recruitment Maneuver in Reducing Shoulder and Upper Abdomen Pain Following Gynecologic Laparoscopic Procedures: A Randomized, Controlled, Triple-Blind Trial. Anesth Pain Med. 2019;9(3). e92444. [PubMed ID: 31497525]. [PubMed Central ID: PMC6712360]. https://doi.org/10.5812/aapm.92444.
-
5.
Norozi V, Ghazi A, Amani F, Bakhshpoori P. Effectiveness of Sublingual Buprenorphine and Fentanyl Pump in Controlling Pain After Open Cholecystectomy. Anesth Pain Med. 2021;11(3). e113909. [PubMed ID: 34540635]. [PubMed Central ID: PMC8438705]. https://doi.org/10.5812/aapm.113909.
-
6.
Rupniewska-Ladyko A, Malec-Milewska M. A High Dose of Fentanyl May Accelerate the Onset of Acute Postoperative Pain. Anesth Pain Med. 2019;9(5). e94498. [PubMed ID: 31903331]. [PubMed Central ID: PMC6935250]. https://doi.org/10.5812/aapm.94498.
-
7.
Asgari Z, Mozafar-Jalali S, Faridi-Tazehkand N, Sabet S. Intraperitoneal dexamethasone as a new method for relieving postoperative shoulder pain after gynecologic laparoscopy. Int J Fertil Steril. 2012;6(1):59-64. [PubMed ID: 25505513]. [PubMed Central ID: PMC4260641].
-
8.
Gayam S, Naduvinakeri K, Rajagopal M, Neelima C. Preoperative dexamethasone and intraperitoneal bupivacaine for postoperative pain relief. Obs Gyne Rev J Obstet Gynecol. 2020;6(4):96-101. https://doi.org/10.17511/joog.2020.i04.05.
-
9.
Wang JJ, Ho ST, Liu YH, Lee SC, Liu YC, Liao YC, et al. Dexamethasone reduces nausea and vomiting after laparoscopic cholecystectomy. Br J Anaesth. 1999;83(5):772-5. [PubMed ID: 10690141]. https://doi.org/10.1093/bja/83.5.772.
-
10.
Ismail EA, Abo Elfadl GM, Bahloul M. Comparison of intraperitoneal versus intravenous dexamethasone on postoperative nausea and vomiting after gynecological laparoscopy: a randomized clinical trial. Korean J Anesthesiol. 2019;72(1):47-52. [PubMed ID: 30223315]. [PubMed Central ID: PMC6369338]. https://doi.org/10.4097/kja.d.18.00132.
-
11.
Assante J, Collins S, Hewer I. Infection Associated With Single-Dose Dexamethasone for Prevention of Postoperative Nausea and Vomiting: A Literature Review. AANA J. 2015;83(4):281-8. [PubMed ID: 26390747].
-
12.
Malik KM, Imani F, Beckerly R, Chovatiya R. Risk of Opioid Use Disorder from Exposure to Opioids in the Perioperative Period: A Systematic Review. Anesth Pain Med. 2020;10(1). e101339. [PubMed ID: 32337175]. [PubMed Central ID: PMC7158240]. https://doi.org/10.5812/aapm.101339.
-
13.
Zhou C, Zhu Y, Liu Z, Ruan L. 5HT3 Antagonists versus Dexamethasone in the Prevention of PONV in Patients Undergoing Laparoscopic Cholecystectomy: A Meta-Analysis of RCTs. Biomed Res Int. 2016;2016:8603409. [PubMed ID: 27891523]. [PubMed Central ID: PMC5116342]. https://doi.org/10.1155/2016/8603409.
-
14.
Yamanaga S, Posselt AM, Freise CE, Kobayashi T, Tavakol M, Kang SM. A Single Perioperative Injection of Dexamethasone Decreases Nausea, Vomiting, and Pain after Laparoscopic Donor Nephrectomy. J Transplant. 2017;2017:3518103. [PubMed ID: 28210502]. [PubMed Central ID: PMC5292178]. https://doi.org/10.1155/2017/3518103.
-
15.
Si XY, Wu LP, Li XD, Li B, Zhou YM. Dexamethasone combined with other antiemetics for prophylaxis after laparoscopic cholecystectomy. Asian J Surg. 2015;38(1):21-7. [PubMed ID: 24942194]. https://doi.org/10.1016/j.asjsur.2014.04.005.
-
16.
Hosseini Valami M, Hosseini Jahromi A, Mahmoodi T, Barikani A. [Comparing the effects of intraperitoneal injection of bupivacaine, morphine, and dexamethasone on pain after elective caesarean section under general anesthesia]. J Maz Univ Med Sci. 2017;27(147):139-49. Persian.
-
17.
Hamilton E, Ravikumar R, Bartlett D, Hepburn E, Hwang MJ, Mirza N, et al. Dexamethasone reduces emesis after major gastrointestinal surgery (DREAMS). Trials. 2013;14:249. [PubMed ID: 23938028]. [PubMed Central ID: PMC3765230]. https://doi.org/10.1186/1745-6215-14-249.
-
18.
Holte K, Kehlet H. Perioperative Single-Dose Glucocorticoid Administration: Pathophysiologic Effects and Clinical Implications. J Am Coll Surg. 2002;195(5):694-712. https://doi.org/10.1016/s1072-7515(02)01491-6.
-
19.
Margulis R, Francis J, Tischenkel B, Bromberg A, Pedulla D, Grtisenko K, et al. Comparison of Dexmedetomidine and Dexamethasone as Adjuvants to Ultra-Sound Guided Interscalene Block in Arthroscopic Shoulder Surgery: A Double-Blinded Randomized Placebo-Controlled Study. Anesth Pain Med. 2021;11(3). e117020. [PubMed ID: 34540645]. [PubMed Central ID: PMC8438728]. https://doi.org/10.5812/aapm.117020.
-
20.
Zhu M, Zhou C, Huang B, Ruan L, Liang R. Granisetron plus dexamethasone for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgery: A meta-analysis. J Int Med Res. 2017;45(3):904-11. [PubMed ID: 28436248]. [PubMed Central ID: PMC5536409]. https://doi.org/10.1177/0300060517703276.
-
21.
Elhakim M, Nafie M, Mahmoud K, Atef A. Dexamethasone 8 mg in combination with ondansetron 4 mg appears to be the optimal dose for the prevention of nausea and vomiting after laparoscopic cholecystectomy. Can J Anaesth. 2002;49(9):922-6. [PubMed ID: 12419717]. https://doi.org/10.1007/BF03016875.
-
22.
Feo CV, Sortini D, Ragazzi R, De Palma M, Liboni A. Randomized clinical trial of the effect of preoperative dexamethasone on nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2006;93(3):295-9. [PubMed ID: 16400707]. https://doi.org/10.1002/bjs.5252.
-
23.
Sultan HM, Gaber A, Nassar M, Esmaeil WME. Intraperitoneal hydrocortisone for pain relief after laparoscopic cholecystectomy. Menoufia Med J. 2018;31(1):126.
-
24.
Elsakka A, Elrefai N, Shehata J, Abdel Mawla AG. Postoperative analgesic efficacy of the pulmonary recruitment manoeuvre compared to intraperitoneal hydrocortisone in laparoscopic gynaecological surgeries. Indian J Anaesth. 2021;65(2):115-20. [PubMed ID: 33776085]. [PubMed Central ID: PMC7983833]. https://doi.org/10.4103/ija.IJA_423_20.
-
25.
Lirk P, Thiry J, Bonnet MP, Joshi GP, Bonnet F, Prospect Working Group. Pain management after laparoscopic hysterectomy: systematic review of literature and PROSPECT recommendations. Reg Anesth Pain Med. 2019;44(4):425-36. [PubMed ID: 30914471]. https://doi.org/10.1136/rapm-2018-100024.
-
26.
Mohtadi A, Nesioonpour S, Salari A, Akhondzadeh R, Masood Rad B, Aslani SM. The effect of single-dose administration of dexamethasone on postoperative pain in patients undergoing laparoscopic cholecystectomy. Anesth Pain Med. 2014;4(3). e17872. [PubMed ID: 25237639]. [PubMed Central ID: PMC4165022]. https://doi.org/10.5812/aapm.17872.
-
27.
Ali BH, Hameed AZ, Isa RJ. Comparative Study of Dexamethasone Versus Ondansetron as Adjuvants to the Intra-Peritoneal Irrigation of Bupivacaine for Reducing the Postoperative Pain in Patients Undergoing Elective Laparoscopic Cholecystectomy. Syst Rev Pharm. 2020;11(12):463-8.
-
28.
Zahra AA, Abo-Elenin K, El-Fiky EM, Kasemy ZA, Helwa AM. Intra Peritoneal Instillation of Bupivacaine or Bupivacaine plus Magnesium Sulphate or Bupivacaine plus Dexamethasone on Post-Operative Pain after Laparoscopic Cholecystectomy: A Randomized Controlled Study. Egypt J Hosp Med. 2021;84(1):2655-62. https://doi.org/10.21608/ejhm.2021.189611.
-
29.
Rajnikant K, Bhukal I, Kaloria N, Soni SL, Kajal K. Comparison of Palonosetron and Dexamethasone with Ondansetron and Dexamethasone to Prevent Postoperative Nausea and Vomiting in Patients Undergoing Laparoscopic Cholecystectomy. Anesth Essays Res. 2019;13(2):317-22. [PubMed ID: 31198253]. [PubMed Central ID: PMC6545955]. https://doi.org/10.4103/aer.AER_21_19.
-
30.
Alkaissi A, Dwaikat M, Almasri N. Dexamethasone, metoclopramide, and their combination for the prevention of postoperative nausea and vomiting in female patients with moderate-to-high risk for ponv undergoing laparoscopic surgery. J Evol Med Dent Sci. 2017;6(75):5353-9. https://doi.org/10.14260/Jemds/2017/1162.
-
31.
Nouri B, Arab M, Lotfpour S. Efficacy of Intraperitoneal Dexamethasone Infusion in Reduction of Shoulder Pain and Nausea/Vomiting After Gynecological Laparoscopy. Fertility Gynecology Andrology. 2021;1(1). e115089. https://doi.org/10.5812/fga.115089.
