Abstract
Background:
Spinal cord stimulation (SCS) is an established treatment modality for neuropathic pain. The critical part of this technique is safe access to the epidural space for lead placement. There have been innovations in radiological views, improving access to the epidural space.Objectives:
This study analyzes the adoption of these technical advantages in daily practiceMethods:
We conducted a survey of members in the Spine Intervention Society and American Society of Regional Anesthesia in regard to the practice patterns in SCS therapy. Here we present our findings regarding the use of contralateral oblique (CLO) and lateral views as well direct upper thoracic or cervicothoracic access for SCS lead insertionResults:
A total of 195 unique responses were received between March 20, 2020 and June 26, 2020. Forty-five percent of respondents “always used” the lateral view technique while 15% “always used” CLO view for SCS lead insertion. Overall, sixty-five percent of respondents used the CLO view with varying frequency. Cervical and upper thoracic approach for cervical SCS lead placement is always or often used by 66.8% of the respondents.Conclusions:
A depth view (CLO or lateral) is always used by only 45 - 60% of the respondents and CLO view has been rapidly adopted in clinical practice for SCS lead insertion. Direct cervicothoracic and upper thoracic is the preferred approach for cervical lead placement by the majority.Keywords
Contralateral Oblique Lateral Spinal Cord Stimulation Fluoroscopy Neurological Injury Standard of Care
1. Background
Spinal cord stimulation (SCS) is frequently used and is an effective therapy for intractable neuropathic pain (1, 2). Whereas the primary indications for spinal cord stimulation remain failed back surgery syndrome and complex regional pain syndrome, there is a growing list of other indications for use, including chronic intractable back pain without previous surgery where other treatments have failed (2). With increasing utilization various aspects of neuromodulation are the subject of several recent clinical reports (3-9).
Spinal cord stimulation is considered safe with a very low rate of direct neurological injury. A study by Cameron reported only 1 case of paralysis among 2972 patients. Multiple studies have shown no significant incidence of neurological injury. A recent analysis by Petraglia however reported a risk of spinal cord injury within 30 days after percutaneous lead insertion at 2.35% (10). The study might have overestimated the risk due to the fact that codes used were not guaranteed for accuracy. Neuromodulation Appropriateness Consensus Committee (NACC) guidelines that recommend a responsive patient during SCS lead insertion also bring this study to attention (11). The contributory causes in neurological injury during SCS have not been defined, however in an ASA closed claims data analysis, direct spinal cord injury accounted for 31% of the total cases; in cases where use of fluoroscopy was defined, fluoroscopy was used 76% of the times (12). One of the technical factors that may lead to injury is the limitation of fluoroscopy in terms of a fixed radiological landmark defining the epidural space and clarity of needle tip visualization, especially in the cervicothoracic placement. The lateral view is often inadequate for needle tip visualization (13). The contralateral view is useful for epidural access and has been shown to be superior to the lateral view for consistency of location as well as needle tip visualization during epidural access, both in the cervical and lumbar spine (14, 15). Whereas there are no safety data to support the superiority of this view, it does provide greater ease of access, consistency of location and virtually eliminates false loss of resistance (14, 15).
Consensus opinion of the multidisciplinary working group representing thirteen organizations recommend anteroposterior and lateral or contralateral view for epidural access (16).
In addition to the question of how fluoroscopy is used for SCS lead placement, the other question is as to whether the cervical lead is placed by low thoracic access or upper thoracic and cervicothoracic access. Since the upper thoracic and cervical access can represent a greater challenge, some providers choose to advance the lead from low thoracic region. The disadvantage of this approach is that it is not reliable and sometimes the lead cannot be advanced beyond the cervicothoracic junction because of the limited tensile strength of the lead. Hence this approach is unpredictable, sometimes necessitating a change of operative plans midway through the procedure if this occurs.
We conducted a survey on the practice patterns of clinician in regard to spinal cord stimulation. There is a dearth of reports on how the epidural space is accessed during SCS lead insertion and how much adoption has occurred of the contralateral oblique view in daily practice in this regard. Here we present our findings on technical aspect of epidural access during SCS lead insertion.
2. Objectives
We chose this as a separate topic so as to analyze and discuss this in depth as it is an important facet of SCS therapy. The information from the survey will help practitioners understand how others access the space and provides insight into the rate of adoption of the CLO view.
3. Methods
A survey with questions related to various aspects of spinal cord stimulation practice was approved by the Institutional Review Board. The survey was developed based upon the authors’ opinion of the important clinical questions regarding various aspects of SCS therapy. The survey was then emailed to the membership of the Spine Intervention Society and the American Society of Regional Anesthesia and recipients were invited to respond anonymously by a survey monkey link. We were not able to send this survey to a dedicated neuromodulation society because of logistic reasons. The recipients were informed as to the nature of the survey and asked to respond only once in case they were members of both of the societies.
The four questions related to spinal cord stimulation lead insertion were:
Do you use a lateral view to obtain epidural access?
Do you use a contralateral oblique view (CLO) for epidural access?
Do you use the metal guide wire prior to lead insertion?
For cervical leads do you attempt cervical/upper thoracic access (instead of low thoracic or lumbar access)?
4. Results
The survey was emailed to 2967 members of SIS, with 1259 opening the email, and 3169 members of ASRA with 1477 opening the email. A total of 195 responses were received for question 1 to 3 and 178 responses for question 4 were received between march 20, 2020 and June, 26, 2020. We calculate the response rate based upon the number of recipients who opened the email as 7.1% and 6.5% respectively. We did not make any adjustment in the overlap within the membership as this is unknown. We also did not make any adjustment for the fact that some recipients may not be performing SCS and became aware that the survey was about SCS practice parameters only after they had opened the email as the number of recipients performing SCS procedures is also unknown given that these are multidisciplinary societies
For the lateral view utilization 45.64% reported using this always and 10.26% reported never using this. Of the rest 9.23% used it often and 34.87% used it sometimes (Table 1). Combining this, it is clear that the majority always or often uses lateral view for epidural access. For the CLO view 14.87% reported using this as always with 34.36% reporting never using it. Of the rest, 11.79% use it frequently while 38.97% use it sometimes (Table 2). Combining these, it is clear that a quarter of the recipients are often or always using CLO view. Since there may be overlap between the recipients always using the lateral or CLO view it can be stated that at a minimum 45.64% always use a depth view for epidural access and assuming no overlay then 60% always use the depth view. In regards to using a metal guide (also termed lead blank), this practice seems to have fallen out of favor with only 11.79% always using it while 44.1% never use it (Table 3).
| Never | Sometimes | Often | Always |
|---|---|---|---|
| 20 (10.26) (6.4 - 15.4) | 68 (34.87) (28.2 - 40) | 18 (9.23) (15.6 - 14.2) | 89 (45.64) (38.7 - 52.6) |
| Never | Sometimes | Often | Always |
|---|---|---|---|
| 67 (34.36) (27.7 - 41.5) | 76 (38.97) (32.1 - 46.2) | 23 (11.79) (7.3 - 16.3) | 29 (14.87) (10.2 - 20.7) |
| Never | Sometimes | Often | Always |
|---|---|---|---|
| 86 (44.10) (37.1 - 51) | 73 (37.44) (30.6 - 44.6) | 13 (6.69) (3.2 - 10.2) | 23 (11.79) (7.3 - 16.3) |
In regards to access location for cervical lead placement, 43.26% of the recipients used cervical or upper thoracic access. Of the rest cervical/upper thoracic access was used often, sometimes or never by 23.6%, 18.54% and 14.61% respectively.
5. Discussion
A survey of fluoroscopic views for lead insertion, metal guide prior to lead insertion, and preferred entry point for cervical lead insertion reveals wide variability in practice patterns. We will analyze and discuss the variability below and provide recommendations based upon survey data and literature
5.1. Lateral and Contralateral Oblique View Use for SCS Lead Insertion
The standard for insertion of spinal cord stimulator lead is that the inserted is under radiological control. The anteroposterior (AP) view provides the standard view to mark the point of insertion, for the mediolateral orientation, and to optimize the interlaminar opening. However, this is a 2-dimensional view that does not provide the depth of needle insertion, and thus cannot help to safely guide epidural access especially when there is ambiguity as to the needle location after the laminar edge is crossed. In order to ascertain the needle depth a lateral view or a contralateral oblique view must be chosen and is recommended (16). A depth view (lateral or contralateral oblique) provides reassurance that the needle has not penetrated beyond the radiographic inferred epidural space. This is particularly useful when good resistance cannot be obtained or when the loss of resistance is not readily encountered after the laminar edge is crossed in the AP view. In a comparative study between the lateral and contralateral oblique view the contralateral oblique view was found to provide superior needle tip visualization, better ability to predict the needle trajectory between the lamina, precise radiological landmark (ventral interlaminar line) for the posterior margin of the epidural space and eliminated the false positive loss of resistance (LOR) (14, 15). Furthermore, the CLO view is especially useful in upper thoracic and cervical access where the interlaminar window is narrow, the margin of error very low (small dorsal epidural space), and the lateral view is particularly suboptimal because of shoulder overlap. The contralateral oblique view has also been validated in other larger studies (17).
The survey shows that depth views are frequently used, with 45.6% always using the lateral view and 14.8% always using the CLO view. Given the overlap, it is clear that a depth view is always used by at least 45.6% of the physicians. It is also clear that at least 40% of the clinicians do not always use the depth view and rely primarily on LOR techniques. Even though the CLO view is a recent addition to clinical practice, 65% of the respondents are familiar with its use.
The survey data has two implications; the quick adoption of the CLO view in regular practice is a testament to the inherent advantages of this view as demonstrated in the studies, and secondly, close to a majority do not always use the depth view in routine access to the epidural space. The two likely reasons why depth view is not always used are firstly, the LOR is often readily obtained in AP view especially by seasoned practitioners who may not feel it unreasonable to deploy the additional view especially since lead advancement will further confirm the epidural location. The second reason is that lateral view does not offer any advantages beyond the ability to ascertain that the needle is still superficial. Thus, the fact that the lateral view does not give a precise radiological landmark for the epidural space makes the final stretch of procedure essentially blind and completely dependent upon LOR over several millimeters. Furthermore, the lateral view does not offer any true ability to determine the needle trajectory. Given that CLO view offers procedural advantages in optimizing shallow entry, trajectory projection, very precise radiological landmark, its adoption will likely lead to further routine deployment of the view for epidural access to SCS lead insertion. Comparison of CLO and lateral view for SCS lead insertion is presented in Table 4.
Comparison of CLO and Lateral View for Epidural Access
| CLO | Lateral | |
|---|---|---|
| Radiological Landmark | On the VILL or within 1 - 3 mm of the VILL | Anywhere from spinolaminar junction to articular pillars (cervical) or spinolaminar line to beyond facet lucency (lumbar) |
| Plot point of insertion | Yes | No |
| Shallow angle | Maximal | Does not allow |
| Trajectory projection | Yes | No (cannot see the interlaminar opening) |
| Needle tip visualization | Crisp | Poor in cervical |
| Confirm dorsal epidural placement of lead | Less reliable | Reliable |
| False loss of resistance | Negligible | Significant |
| Ease of placement | Straightforward | Variable |
The other area to explore in relation to the survey findings is the causes and prevention of spinal cord injury during SCS lead insertion. Spinal cord stimulation is considered safe with a very low rate of direct neurological injury. A study by Cameron reported only 1 case of paralysis among 2972 patients (18). Multiple studies have shown no significant incidence of neurological injury. A recent study looking at billing data reported a risk of spinal cord injury within 30 days after percutaneous lead insertion at 2.35% (10). Furthermore, ASA closed claims database from 2005 - 2008 identified direct spinal cord injury from needle trauma as the primary cause in 31% of the cases. It was not possible to discern whether fluoroscopy was used in all cases, but among the cases where it was discernible, fluoroscopy use was evident 76% of times where neurological injury occurred (12). Thus, mere use of fluoroscopy will not guard against improper use or limitations of fluoroscopy. It is possible that underutilization of depth views or inadequacy of the lateral view in the cervical spine may be the drivers of neurological injury during SCS.
In terms of standard of care, significant proportion do not always use depth view for epidural access even though it is recommended by multi-society group. In terms of best practices, given the data on risk of nerve injury, a depth view must be always used or quickly deployed in case of ambiguity. Among depth views CLO views offer clear advantages over the lateral view.
Since the contralateral oblique view is a relatively new addition, we will briefly describe the steps in cervical and thoracic SCS lead insertion based upon the studies and our practice
5.2. Cervical Lead Placement with Access from C7-T4 Levels
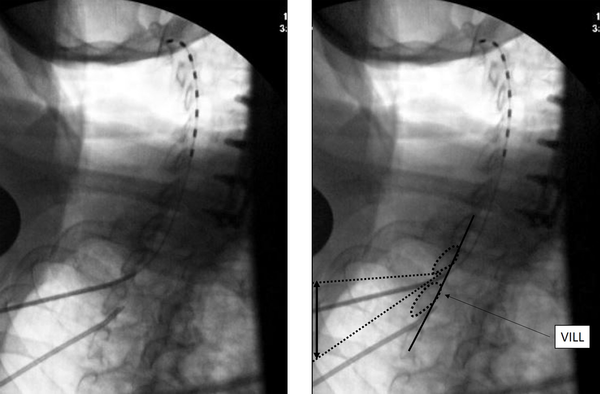
The needle insertion point is marked about half to one level below the laminar margin. Once a 14G SCS introducer needle is introduced to the top of the inferior lamina, a CLO view at 50 degrees is taken to the contralateral side for depth of needle insertion and trajectory, aiming for the superior edge of the inferior lamina. Loss of resistance is initiated just before the ventral interlaminar line (VILL) and loss expected at, or within 1-3 mm of the VILL. The most important aspect of this technique is to use an optimal obliquity of 50 degrees, or close to it, as the needle tip position depends on obliquity. Other important aspects are to visualize the VILL clearly, not the foramen, and to not advance greater than 2 - 3 mm beyond the VILL without checking other views, clearing the needle, passing the lead and other such maneuvers. The loss can be subtle, and if the needle tip has crossed the VILL lead insertion may be attempted even though the loss has not occurred, as the tip may already be in the epidural space. Lesser degrees of obliquity from the AP make the needle appear deeper to the VILL, thus most needle tips will be in Zone 3 at an obliquity of 40 degrees (14). Lateral view is helpful in confirming dorsal epidural space lead localization. The SCS lead placement in CLO view is shown in Figure 1.
The upper end of the lead lies at C2, and the black double arrow represents how much the needle trajectory may be adjusted when using this view. VILL: ventral interlaminar line.
5.3. Thoracic Lead Placement Using the High Lumbar or Low Thoracic Approach
The steps of lead placement are exactly the same except the point of insertion is 1 - 2 levels below, instead of half to one level below. The advantage of the view is the ability to plot a shallower angle of insertion for lead placement. If an AP view is being used for lead insertion, and there is no resistance, or the needle cannot be advanced, a quick view can instantly clarify the anatomy and remove all the guesswork.
5.4. Metal Guide Prior to Lead Insertion
The guide wire or lead blank is no longer considered by most to be an essential step before insertion of the lead. The lead blank was initially proposed as a simple device to confirm the epidural space, create a pathway for the lead by ensuring its easy passage into the epidural space and confirm that there is no paresthesia elicited because of its narrower diameter compared to the lead. There have been significant advances in epidural needle access techniques and lead design including steerability that may contribute to the low use of the guide wire. There are no studies evaluating its use in the SCS procedure. The findings of the survey are consistent with decreasing use of the guide wire with only 18.3% of the respondents always or often using guide wire prior to lead insertion.
5.5. Cervical and Upper Thoracic Access for Cervical Lead Placement
Cervical Leads can be placed by upper lumbar low thoracic access and often this is the preferred route given the simpler approach that is more practiced and familiar. However, one of the major drawbacks with this approach is that often it is not possible to advance and guide the lead, especially when targeting the upper cervical levels. With the long distance the lead often becomes non-steerable or bends proximally on pushing, as it does not have enough tensile strength to traverse this region. This tends to happen at the cervicothoracic junction but not always. Neck lordosis and cervicothoracic curvature often needs to be minimized when facilitating this passage. Additionally, the ability to pass the lead during the trial does not guarantee that the same will happen during permanent placement.
When the lead cannot be advanced to the upper cervical region, the operative course has to be changed and the case is either abandoned or altered to high thoracic epidural access.
The major reason behind the reluctance for high thoracic and cervicothoracic access is the difficulty in needle tip visualization when close to the epidural space and the low margin of error for excessive advancement. The situation where the needle is being advanced beyond the laminar edge and loss of resistance has not yet been obtained can be anxiety provoking. This together with the fact that cervical procedures are associated with higher incidence of complications explains the desire to avoid accessing the epidural space in the upper region of the spine.
In looking at the Table 5, 67% of the recipients always or often perform cervical or high thoracic access for cervical lead placement with only 15% avoiding this approach altogether.
There are no studies that explore this technical aspect of SCS lead insertion into the cervical spine, and NACC does not provide any guidance. Given the survey data, the limitations of lumbar and low thoracic access for cervical lead placement, as well as introduction of the CLO view improving visualization and precision, it may be best practice to access the high thoracic area preferentially rather than use this approach secondarily. However, using this as a backup approach, especially if there is less or no familiarity with the CLO view, would also be a reasonable option, as practiced by 18.5% of the recipients.
For Cervical Leads Do You Attempt Cervical/Upper Thoracic Access? (Instead of Lumbar Access)? 178 Responses a
| Never | Sometimes | Often | Always |
|---|---|---|---|
| 26 (14.61) (7.3 - 16.3) | 33 (18.54) (12.8 - 24.3) | 42 (23.60) (17.4 - 29.8) | 77 (43.26) (36 - 50.5) |
There are several limitations to this study. First, the limitations relate to recall bias associated with a survey like this and secondly the true response rate is unknown. However the true response rate is likely much higher because of the overlap in the membership of the societies and the recipients were asked to fill the survey only once. Additionally proportion of members performing SCS procedure is unknown and only the recipients performing SCS would be true recipients. The strength of this survey is that this is the first report on SCS practice in a large cohort of practicing neuromodulation physicians who are also active members of academic societies.
5.6. Conclusions
The practice patterns for SCS lead insertion reveals significant physician variability. A depth view is always used by 45 - 60% of the respondents. CLO view which is relatively new compared to the lateral has also gained adoption into practice with 26.5% of the respondents using it always or often and 65% of the recipients reporting utilization of this view. Cervical and upper thoracic approach for cervical SCS lead placement is used always or often by 66.8% of the respondents. The true incidence and frequency of causes behind neurological injury in SCS is unknown but given the ASA closed claims data direct mechanical injury during lead insertion might be an important variable. It is likely that these innovations may reduce the number of neurological injuries related to technical aspects of epidural access but given the low incidence, this is difficult to study and identify. The radiologic view is even more important when leads are placed anatomically or under intraoperative neuromonitoring in which case deep sedation or general anesthesia may be utilized. Future guidelines to minimize neurological complications during SCS must take these advances into consideration and make recommendation for clinical practice as well as the need to train future generations in best and safe methods of SCS lead insertion.
References
-
1.
Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762-70. discussion 770. [PubMed ID: 18981888]. https://doi.org/10.1227/01.NEU.0000325731.46702.D9.
-
2.
Al-Kaisy A, Van Buyten JP, Kapural L, Amirdelfan K, Gliner B, Caraway D, et al. 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: subanalysis of pooled data from two prospective studies. Anaesthesia. 2020;75(6):775-84. [PubMed ID: 32383509]. [PubMed Central ID: PMC7384077]. https://doi.org/10.1111/anae.15036.
-
3.
Amorizzo E, Colini-Baldeschi G. Peripheral Nerve Stimulation: Two Cases of Intractable Neuropathic Pain. Anesth Pain Med. 2021;11(2). e113162. [PubMed ID: 34336623]. [PubMed Central ID: PMC8314092]. https://doi.org/10.5812/aapm.113162.
-
4.
Berger AA, Urits I, Hasoon J, Gill J, Aner M, Yazdi CA, et al. Improved Pain Control with Combination Spinal Cord Stimulator Therapy Utilizing Sub-perception and Traditional Paresthesia Based Waveforms: A Pilot Study. Anesth Pain Med. 2021;11(1). [PubMed ID: 34221951]. [PubMed Central ID: PMC8241823]. https://doi.org/10.5812/aapm.113089.
-
5.
Berger AA, Liu Y, Possoit H, Rogers AC, Moore W, Gress K, et al. Dorsal Root Ganglion (DRG) and Chronic Pain. Anesth Pain Med. 2021;11(2). [PubMed ID: 34336621]. [PubMed Central ID: PMC8314073]. https://doi.org/10.5812/aapm.113020.
-
6.
Abejon D, Vancamp T, Monzon EM. A Cost-Consequence Analysis Examining the Differences Between Non-Rechargeable and Rechargeable Systems. Anesth Pain Med. 2020;10(1). e100308. [PubMed ID: 32337173]. [PubMed Central ID: PMC7158244]. https://doi.org/10.5812/aapm.100308.
-
7.
Urits I, Schwartz R, Smoots D, Koop L, Veeravelli S, Orhurhu V, et al. Peripheral Neuromodulation for the Management of Headache. Anesth Pain Med. 2020;10(6). [PubMed ID: 34150578]. [PubMed Central ID: PMC8207880]. https://doi.org/10.5812/aapm.110515.
-
8.
Papa A, Di Dato MT, Buonavolonta P, Saracco E, Salzano AM, Casale B. Clinical Management of Il-6 Driven Cytokine Storm Related to COVID-19 in a Patient with Recent Spinal Cord Stimulator Implants: A Case Report. Anesth Pain Med. 2020;10(4). e104151. [PubMed ID: 33134148]. [PubMed Central ID: PMC7539055]. https://doi.org/10.5812/aapm.104151.
-
9.
Sarrafpour S, Hasoon J, Urits I, Viswanath O, Mahmoudi K, Simopoulos TT, et al. Antibiotics for Spinal Cord Stimulation Trials and Implants: A Survey Analysis of Practice Patterns. Anesth Pain Med. 2021;11(5). e120611. [PubMed ID: 35075422]. [PubMed Central ID: PMC8782197]. https://doi.org/10.5812/aapm.120611.
-
10.
Petraglia 3rd FW, Farber SH, Gramer R, Verla T, Wang F, Thomas S, et al. The Incidence of Spinal Cord Injury in Implantation of Percutaneous and Paddle Electrodes for Spinal Cord Stimulation. Neuromodulation. 2016;19(1):85-90. [PubMed ID: 26644210]. [PubMed Central ID: PMC4724311]. https://doi.org/10.1111/ner.12370.
-
11.
Deer TR, Lamer TJ, Pope JE, Falowski SM, Provenzano DA, Slavin K, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Safety Guidelines for the Reduction of Severe Neurological Injury. Neuromodulation. 2017;20(1):15-30. [PubMed ID: 28042918]. https://doi.org/10.1111/ner.12564.
-
12.
Rathmell JP, Michna E, Fitzgibbon DR, Stephens LS, Posner KL, Domino KB. Injury and liability associated with cervical procedures for chronic pain. Anesthesiology. 2011;114(4):918-26. [PubMed ID: 21386702]. https://doi.org/10.1097/ALN.0b013e31820fc7f2.
-
13.
Abbasi A, Malhotra G. The "swimmer's view" as alternative when lateral view is inadequate during interlaminar cervical epidural steroid injections. Pain Med. 2010;11(5):709-12. [PubMed ID: 20353409]. https://doi.org/10.1111/j.1526-4637.2010.00829.x.
-
14.
Gill JS, Aner M, Nagda JV, Keel JC, Simopoulos TT. Contralateral oblique view is superior to lateral view for interlaminar cervical and cervicothoracic epidural access. Pain Med. 2015;16(1):68-80. [PubMed ID: 25220833]. https://doi.org/10.1111/pme.12557.
-
15.
Gill JS, Nagda JV, Aner MM, Keel JC, Simopoulos TT. Contralateral Oblique View Is Superior to the Lateral View for Lumbar Epidural Access. Pain Med. 2016;17(5):839-50. [PubMed ID: 26814266]. https://doi.org/10.1093/pm/pnv031.
-
16.
Rathmell JP, Benzon HT, Dreyfuss P, Huntoon M, Wallace M, Baker R, et al. Safeguards to prevent neurologic complications after epidural steroid injections: consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology. 2015;122(5):974-84.
-
17.
Derby R. Reliability and Safety of Contra-Lateral Oblique View for Interlaminar Epidural Needle Placement. Pain Physician. 2017;1(21;1):E65-73. https://doi.org/10.36076/ppj.2017.1.E65.
-
18.
Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100(3 Suppl Spine):254-67. [PubMed ID: 15029914]. https://doi.org/10.3171/spi.2004.100.3.0254.