Abstract
Context:
This systematic review and meta-analysis evaluated the effect of the intra-articular injection of platelet-rich plasma (PRP) and oxygen-ozone therapy and provided an evidence-based methodology to treat KOA.Method:
Databases, including Cochrane Library, PubMed, and EMBASE, were searched. The retrieval period was before 2021. Two reviewers performed the process of screening and data extraction. Mean differences were calculated [95% confidence interval (CI)] with an inverse-variance method and fixed effect model. Meta-analysis was performed using the latest version of STATA version 16.Results:
A total of 12 studies out of 769 articles were evaluated. The mean difference of visual analog scale score between ozone and control groups in the first month after injection was -0.02 (MD, -0.02; 95% CI: -0.32, 0.28; P < 0.05). Mean differences of WOMAC pain, stiffness, and physical function score between baseline and after PRP were -3.53 (MD: -3.53; 95% CI: -4.04, -3.02; P = 0.00), -0.60 (MD: -0.60; 95% CI: -4.0 - 0.864, -0.34; P = 0.00), and -5.96 (MD: -5.96; 95% CI: -7.83, -4.09; P = 0.00).Conclusions:
Our results showed that to treat knee osteoarthritis, using PRP for a longer period of 6 - 12 months after the intervention shows better clinical results, while oxygen-ozone therapy has short-term results.Keywords
Oxygen-Ozone Therapy Platelet-Rich Plasma Knee Osteoarthritis
1. Context
Knee osteoarthritis (KOA) may be a predominant knee degenerative disorder in which cartilage degeneration, joint instability, subchondral bone hyperplasia, impaired function, and knee pain are observed (1). KOA considerably influences the quality of life and may be a broad public well-being concern (2). The incidence of KOA in the United States has doubled since the mid-twentieth century (3). This disease has caused a significant negative effect on people’s performance. The first-line treatment for KOA recommended by the Osteoarthritis Society International (OARSI) is conservative (4). Non-pharmacologic conservative treatments include muscle exercise and general exercise. However, non-drug methods are mainly challenging to control (5). Corticosteroid injections, analgesics, and nonsteroidal anti-inflammatory drugs (NSAIDs) are the primary pharmacologic treatments. Although the mentioned medications have been successful, they have side effects (6).
Recently, considerable studies have been conducted on the intra-articular injection of platelet-rich plasma (PRP) and oxygen-ozone therapy for KOA treatment. Many investigations reported that the intra-articular injection of PRP and oxygen-ozone therapy could alleviate pain in patients with KOA (7, 8). Research showed that PRP might be a useful treatment for mild to moderate KOA (9). The ozone (O3) structure is gaseous, consisting of three oxygen atoms (10). Based on the existing literature, it has been predicted that a suitable oxygen-ozone mixture can reduce pain, have protective effects on cartilage, and decrease oxidative stress. Therefore, it can be used as an alternative to other injection methods (11).
2. Objective
This systematic review and meta-analysis investigated the effect of the intra-articular injection of PRP and attempted to provide an evidence-based methodology to treat KOA.
3. Methods
The present study is based on the PRISMA guideline used for systematic studies and meta-analysis (12), along with a patient, intervention, comparison, and outcome (PICO) strategy to answer the research questions. The databases PubMed, Cochrane Library, and EMBASE were searched. The retrieval period was papers published until 2021. Two reviewers performed the process of screening and data extraction. The MeSH terms searched on PubMed were ["Platelet-Rich Plasma"(Mesh) AND "Ozone"(Mesh)] AND ["Osteoarthritis"(Mesh) OR "Osteoarthritis, Knee"(Mesh)] AND ["Knee Joint"(Mesh) OR "Knee"(Mesh) OR "Osteoarthritis, Knee/classification"(Mesh) OR "Osteoarthritis, Knee/diagnosis"(Mesh) OR "Osteoarthritis, Knee/diagnostic imaging"(Mesh) OR "Osteoarthritis, Knee/drug therapy"(Mesh) OR "Osteoarthritis, Knee/radiotherapy"(Mesh) OR "Osteoarthritis, Knee/surgery"(Mesh) OR "Osteoarthritis, Knee/therapy"(Mesh)].
Other databases were searched for the keywords “PRP”, “platelet-rich plasma”, “knee osteoarthritis”, “KOA”, “Ozone therapy”, and “ozone injection”.
3.1. Inclusion and Exclusion Criteria
3.1.1. Inclusion Criteria
Randomized controlled trials (RCT), cohort studies, and clinical trials performed on oxygen-ozone therapy, and PRP in humans published in English were included.
3.1.2. Exclusion Criteria
The exclusion criteria entailed meeting abstracts, review articles, in vitro studies, case reports, duplicate publications, information overlap, and articles with incomplete data.
3.1.3. PICO Strategy
- Patients: Patients with a precise diagnosis of KOA
- Interventions: Intra-articular injection of PRP and oxygen-ozone therapy
- Comparison: Control group
- Outcome: WOMAC score, pain, stiffness, and physical function
3.2. Study Selection, Data Extraction, and Analysis Method
The extracted data in this investigation were author name, distribution year, sample size, mean age, intervention measures, radiographic classification, and outcome indicators of the clinical effect of the intervention. Newcastle-Ottawa Scale (NOS) (13) was applied to assess the quality and the risk of the bias of cohort studies. Three measurements are measured on this scale (determination, outcome, and comparability of cohorts) with nine items this scoring is graded as: 1 - 3, low; 4 - 6, medium; 7 - 9, high quality.
The Cochrane collaboration tool (14) was utilized to evaluate the quality and the risk of the bias of RCTs. The score of this scale was 1 for low risk and 0 for high and uncertain risk. Scale scores are 0 - 6, with a higher score showing higher quality. Mean differences (95% CI) with an inverse-variance method and fixed effect model were determined. Random effects were also examined for addressing potential heterogeneity, with a coefficient of I2 indicating heterogeneity and a coefficient of more than 50% indicating moderate to high heterogeneity. Meta-analysis was performed using the STATA version 16.
4. Results
4.1. Searching Database
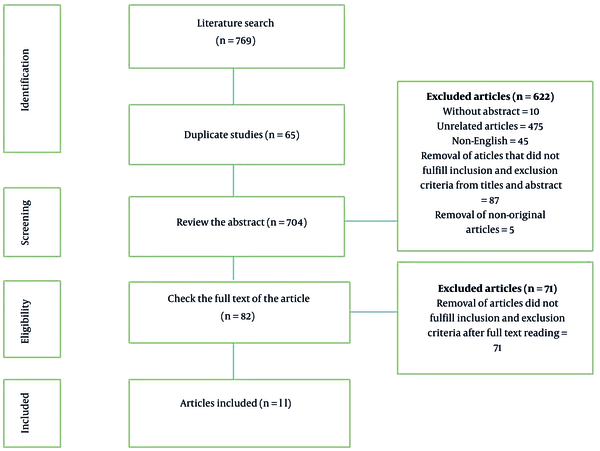
First, the search was performed using primary keywords, and 769 studies were found in the database. After removing the duplicate studies found in the databases, 703 studies were selected to review the abstracts. Following studying the abstracts based on the inclusion and exclusion criteria, 622 papers were removed, and the full texts of 82 articles were reviewed. Next, 71 papers were deleted at this stage, and only 11 studies were selected to be included in the current meta-analysis (Figure 1).
PRISMA flowcharts
4.2. Characteristics of Studies
4.2.1. Oxygen-Ozone Therapy
Six RCTs were selected for meta-analysis and systematic review. The total number of cases in the ozone group was 251 (53 male and 198 female), with a mean age of 63.75 ± 7.11 years. The total number of cases in the control group was 235 (60 male and 175 female), with a mean age of 59.57 ± 6.9 years (Table 1).
Demographic and Clinical Information on RCTs
| Variables | Study | Study Design | Number of Participants | Age (Mean ± SD) | Scale | Groups | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Control | Intervention | ||||||
| Male | Female | Male | Female | ||||||||
| Oxygen-ozone Therapy | Babaei-Ghazani et al. (15) | RCT | 3 | 28 | 7 | 24 | 59.65 ± 10.2 | 56.26 ± 7.8 | WOMAC, VAS, ROM, effusion on US images | Corticosteroids | Ozone |
| Raeissadat et al. (16) | RCT | 17 | 50 | 18 | 56 | 58.1 ± 6.4 | 38.5 ± 8 | VAS and Lysholm score | HA | Ozone | |
| Feng and Beiping (17) | RCT | 15 | 20 | 18 | 23 | 64.57 ± 6.7 | 62.2 ± 7.5 | VAS and Lysholm score | Celecoxib and glucosamine | Ozone | |
| Lopes de Jesu et al. (18) | RCT | 8 | 53 | 9 | 26 | 70.5 ± 7.2 | 69.5 ± 7.6 | VAS, SF-36, | Placebo | Ozone | |
| Duymus et al. (19) | RCT | 4 | 31 | 1 | 33 | 59.4 ± 5.7 | 60.3 ± 5.1 | VAS and WOMAC score | HA | Ozone | |
| Invernizzi et al. (20) | RCT | 6 | 16 | 7 | 13 | 70.3 ± 6.5 | 70.7 ± 5.4 | VAS, OKQ, SF-12, and EuroQoL | HA | Ozone | |
| Platelet-rich plasma | Rahimzadeh et al. (22) | RCT | 21 | 21 | 65.5 ± 6.64 | 64.3 ± 5.31 | WOMAC Scores | Prolotherapy | PRP | ||
| Yu et al. (23) | RCT | 50 | 54 | 48 | 40 | 46.2 ± 8.6 | 51.5 ± 9.3 | WOMAC, AE | HA | PRP | |
| Duymus et al. (19) | RCT | 4 | 31 | 1 | 33 | 59.4 ± 5.7 | 60.3 ± 5.1 | VAS and WOMAC score | HA | PRP | |
| Rahimzadeh et al. (24) | RCT | 15 | 12 | 13 | 14 | 63.67 ± 9.6 | 61.26 ± 4.2 | WOMAC Scores | PRP and Somatropin | PRP | |
| Abate et al. (25) | RCT | 21 | 19 | 31 | 9 | 60.9 ± 9 | 56.7 ± 11.2 | VAS, AE | PRP and HA | PRP | |
| Rahimzadeh et al. (26) | RCT | 11 | 13 | 9 | 11 | 56.95 ± 8.3 | 61.15 ± 7.4 | VAS Scores | Erythropoietin | PRP | |
4.2.2. Platelet-Rich Plasma
Six RCTs were selected for the systematic review and meta-analysis. The total number of cases in the PRP group was 251 (112 male and 139 female), with a mean age of 58.77 ± 5.12 years. The number of cases in the control group was 230 (113 male and 117 female) with a mean age of 59.20 ± 6.5 years (Table 1).
4.3. Bias Assessment
Reviewing the quality of studies by the Cochrane Collaboration tool revealed that all selected studies had a low risk of bias. Therefore, the quality of studies was regarded as high (Figure 2) (15-26).
Risk of bias assessment [Cochrane Collaboration’s tool (14)]
![Risk of bias assessment [Cochrane Collaboration’s tool (14)] Risk of bias assessment [Cochrane Collaboration’s tool (14)]](https://services.brieflands.com/cdn/serve/315e4/86d6e934a88fad45a30083983777d2526eae9338/aapm-12-4-127121-i002-preview.png)
4.4. VAS Score of the Ozone and Control Groups On the First and Sixth Months Post-injection
Mean differences of VAS score between the ozone and control groups on the first month after injection was -0.02 (MD: -0.02; 95% CI: -0.32, 0.28; P < 0.05) (I2 = 87.26%; P = 0.00). A significant difference was found between the ozone and control groups (Figure 3A).
A, Forest plot showed the VAS score of the ozone and control groups in the first month after injection; B, Forest plot showed the VAS score of the ozone and control groups on the sixth month following the injection.
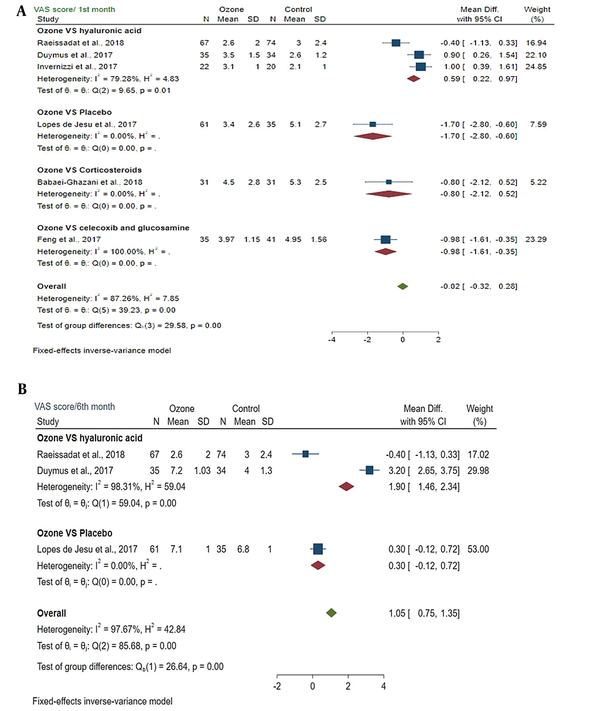
4.4.1. Subgroup Meta-analysis
The test of subgroup differences between groups showed a statistically significant difference (P = 0.00). VAS score was reduced in the ozone and control groups.
Mean differences of VAS score between ozone and control groups on the sixth month after injection was 1.05 (MD: 1.05; 95% CI: 0.75, 1.35; P > 0.05) (I2 = 97.67%; P = 0.00). No significant difference was found between the ozone and control groups (Figure 3B).
4.5. WOMAC Score of the Ozone and Control Groups on the First, Third, Sixth, and Ninth Months Following Injection
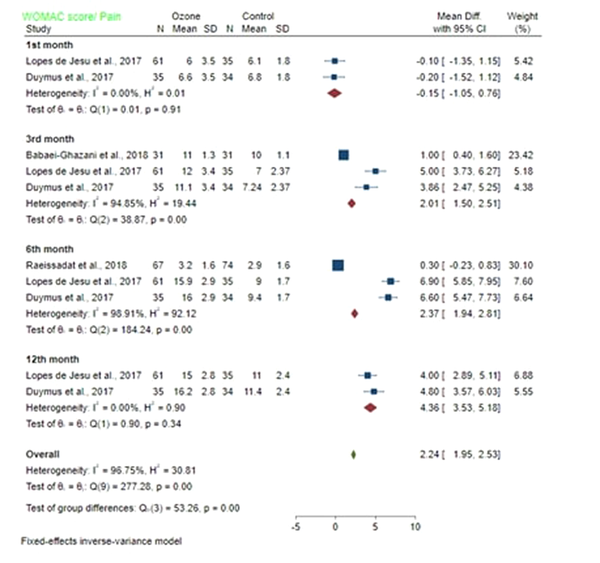
4.5.1. Pain
The mean difference of WOMAC/pain score between the ozone and control groups in the first month after injection was -0.15 (MD: -0.15; 95% CI -1.05, 0.76; P < 0.05) with low heterogeneity (I2 = 0%; P = 0.91). A significant difference was found between the pain of ozone and control groups. The mean difference of WOMAC/pain score between the ozone and control groups in the third month after injection was 2.01 (MD: 2.01; 95% CI 1.5, 2.5; P < 0.05) with high heterogeneity (I2 = 94.85%; P = 0.00). Pain in the ozone group was significantly different from the control group. The mean difference of WOMAC/pain score between the ozone and control groups on the sixth month post-injection was 2.37 (MD: 2.37; 95% CI: 1.94, 2.81; P > 0.05) with high heterogeneity (I2 = 98.91%; P = 0.00). No significant difference was found in pain between the ozone and control groups. The mean difference of WOMAC/pain score between ozone and control groups on the 12th month after injection was 4.36 (MD: 4.36; 95% CI: 3.53, 5.18; P > 0.05) with a low heterogeneity (I2 = 0%; P = 0.34). Pain was not significantly different between the ozone and control groups (Figure 4).
Forest plot showed WOMAC score/pain of the ozone and control groups on the first, third, sixth, and twelfth month following injection.
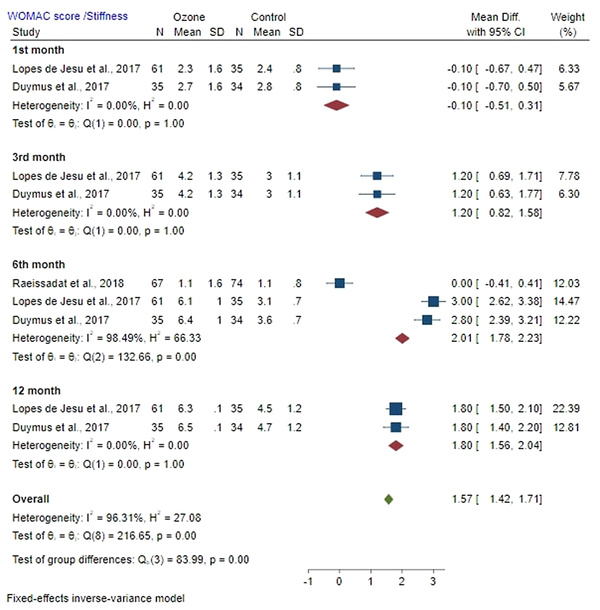
4.5.2. Stiffness
Mean difference of WOMAC stiffness score between the ozone and control groups on the first month after injection was -0.10 (MD: -0.10; 95% CI: -0.51, 0.31; P < 0.05) with a low heterogeneity (I2 = 0%; P = 1.00). A significant difference was found in stiffness between the ozone and control groups.
Mean difference of WOMAC stiffness score between the ozone and control groups on the third month post-injection was 1.20 (MD: 1.20; 95% CI: 0.82, 1.58; P < 0.05) with a low heterogeneity (I2 = 0%; P = 1.00). A significant difference was found in stiffness between the ozone and control groups. The mean difference of WOMAC stiffness score between the ozone and control groups in the sixth month after injection was 2.01 (MD: 2.01; 95% CI: 1.78, 2.23; P > 0.05) with high heterogeneity (I2 = 98.94%; P = 0.00). Stiffness was not significantly different between the ozone and control groups. Mean difference of WOMAC stiffness score between the ozone and control groups on the 12th month post-injection was 1.80 (MD: 1.80; 95% CI: 1.56, 2.04; P > 0.05) with a low heterogeneity (I2 = 0%; P = 1.00). No significant difference was observed in stiffness between the ozone and control groups (Figure 5).
Forest plot showed WOMAC score/Stiffness of the ozone and control groups on the first, third, sixth, and twelfth month after injection.
4.6. WOMAC Score on the Baseline and After PRP
4.6.1. Pain
Mean difference of WOMAC pain score between baseline and after PRP was -3.53 (MD: -3.53; 95% CI: -4.04, -3.02; P = 0.00) with a high heterogeneity (I2 = 99.19%; P = 0.00). Baseline pain and post-PRP pain were significantly different (Figure 6A).
Forest plot showed: A, WOMAC score/pain between baseline and after PRP; B, WOMAC score/stiffness between baseline and after PRP; C, WOMAC score/physical function between baseline and after PRP.
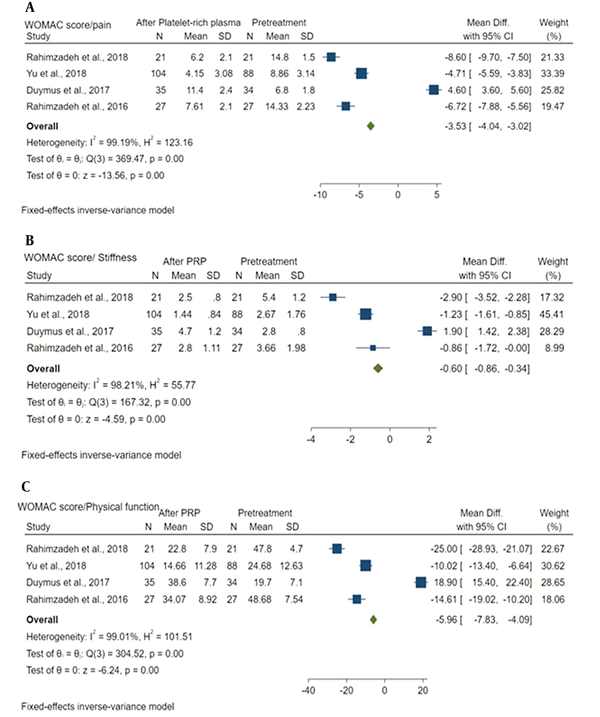
4.6.2. Stiffness
Mean difference of WOMAC stiffness score between baseline and after PRP was -0.60 (MD: -0.60; 95% CI: -4.0 - 0.864, -0.34; P = 0.00) with a high heterogeneity (I2 = 98.21%; P = 0.00). A significant difference was detected in stiffness between the baseline and after PRP (Figure 6B).
4.6.3. Physical Function
Mean difference of WOMAC physical function score between baseline and after PRP was -5.96 (MD: -5.96; 95% CI: -7.83, -4.09; P = 0.00) with a high heterogeneity (I2 = 99.01%; P = 0.00). A significant difference was observed in physical function between the baseline and post-PRP (Figure 6C).
5. Discussion
KOAs reduce patients' quality of life and have psychological and physical effects on elderly patients (27). KOA is gradually becoming prevalent, especially in the elderly population, and is a challenge that has become a serious health issue worldwide (28). No conservative treatment has been reported for KOA (29). Although surgery can be an effective treatment, it can only be applied to cases with severe KOA (30). PRP and oxygen-ozone therapy for KOA may be expensive and complicated. Moreover, the recovery period in surgical treatment is long, and there are some unavoidable risks and complications (31). Therefore, further investigations are required to assess novel strategies and treatments for KOA. PRP and oxygen-ozone therapy have recently received much attention for KOA treatment (32, 33). PRP is prepared by centrifuging autologous blood, and the platelet level can be increased approximately 10-fold, which consists of about 1500 proteins resulting in the release of growth factors and macrophages following activation, which reduces the inflammatory response and articular cartilage repair and regeneration (34, 35). Oxygen-ozone therapy can be beneficial in KOA management. When injected into the knee, reactive oxygen species and lipid oxidation products are produced. The resulting molecules inhibit or reduce the proteolytic enzymes and diminish the release of proinflammatory cytokines (36).
We evaluated the efficacy of oxygen-ozone therapy and PRP in treating KOA. To assess the effectiveness of oxygen-ozone therapy, it was compared with interventions, such as hyaluronic acid (HA) injections, placebo, and medications. The present meta-analysis showed that oxygen-ozone therapy is effective for a short time, especially in 1 - 3 months after oxygen-ozone therapy. However, after one year, it is not different from the control group. Six months following oxygen-ozone therapy, its therapeutic efficacy decreased. It is noteworthy that these findings were observed in all WOMAC subscales and VAS scores. However, due to the high heterogeneity between studies, investigations should be conducted with the same procedure and similar follow-up periods to confirm this evidence. Duymus et al. in 2017 (19) showed that ozone was significantly less effective than HA after three months, and ozone efficacy disappeared 6 months after injection, while the clinical effect of HA continued. Raeissadat et al. in 2018 conducted a systematic review and meta-analysis, reporting similar results to the findings of the present study (37). Sconza et al. (38), in 2020, in a systematic review of RCTs, evaluated eleven RCTs and reported oxygen-ozone therapy as a safe approach with incentive effects on pain control and short-term performance improvement. Another randomized, single-blind study was performed by de Sire et al. on the long-term influence of intra-articular oxygen-ozone therapy compared with HA in patients with KOA. Out of 42 patients, 22 underwent O2O3, and 20 were in the HA group. Both O2O3 and HA groups showed a significant reduction in VAS, while the HA group had significantly lower VAS (39). Paolucci et al. evaluated the effect of focal vibration and intra-articular oxygen-ozone therapy in KOA. The MRC, VAS, and KOOS scores significantly improved in the O2O3-mFV compared to the O2O3 group. The analysis demonstrated that all scores were better over time compared to the baseline, even one month post-treatment (40).
Present meta-analysis demonstrated that PRP has a long-term effect. Hohmann et al. (41), in a meta-analysis and systematic review in 2020, suggested that PRP was superior to HA for symptomatic knee pain in 6 and 12 months. A systematic review of the literature comparing the safety and efficacy of PRP reported that PRP had long-term effectiveness (42). The results of the studies are consistent with the present investigation. However, due to the high heterogeneity between RCT studies, which has been noted in all previous studies, research with similar methodologies and follow-up periods are required.
Bennell et al. reviewed the studies on PRP in 2012, and 15 RCTs in KOA and three in hip OA that compared PRP with other intra-articular injection therapeutic methods were evaluated. Overall, the results of this investigation showed that PRP could be used as a safe treatment that can offer symptomatic benefits for OA, specifically in a short period. Patients with less severe osteoarthritis who were younger were more responsive. They found that no definitive conclusions could be made from these 15 RCTs about the effects of PRP on OA (43). Gilat et al. conducted a study on the effect of HA and PRP on KOA. These authors found that both PRP and HA were effective in the treatment of symptomatic KOA. HA injections could provide short-term improvement, while PRP could offer better therapeutic relief, specifically with the administration of leukocyte-poor (LP-PRP) formulations. According to the limited available information, various formulations of HA-PRP conjugates are predicted to offer a synergistic effect, leading to a clinically considerable improvement in both function and pain (44). The present study had some limitations, such as differences in follow-up period that caused the results to be different, variable research methods, the different control groups in the studies, high heterogeneity between the findings, and small sample size in some studies. Future investigations should be performed with the same methodology, a control group similar to other studies, and a comparison of oxygen-ozone therapy and PRP.
5.1. Conclusions
According to the findings of the current study, for treating KOA, using PRP for a longer period of 6 - 12 months post-intervention shows better clinical results. On the other hand, oxygen-ozone therapy has short-term outcomes. Therefore, it can be stated that PRP is a superior treatment to oxygen-ozone therapy in the treatment of KOA. Further RCTs comparing PRP versus oxygen-ozone therapy are needed to confirm the present evidence.
Acknowledgements
References
-
1.
Pereira D, Peleteiro B, Araújo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011;19(11):1270-85. [PubMed ID: 21907813]. https://doi.org/10.1016/j.joca.2011.08.009.
-
2.
Williams QI, Gunn AH, Beaulieu JE, Benas BC, Buley B, Callahan LF, et al. Physical therapy vs. internet-based exercise training (PATH-IN) for patients with knee osteoarthritis: study protocol of a randomized controlled trial. BMC Musculoskelet Disord. 2015;16:264. [PubMed ID: 26416025]. [PubMed Central ID: PMC4587879]. https://doi.org/10.1186/s12891-015-0725-9.
-
3.
Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A. 2017;114(35):9332-6. [PubMed ID: 28808025]. [PubMed Central ID: PMC5584421]. https://doi.org/10.1073/pnas.1703856114.
-
4.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578-89. [PubMed ID: 31278997]. https://doi.org/10.1016/j.joca.2019.06.011.
-
5.
No authors listed. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905-15. [PubMed ID: 11014340]. https://doi.org/10.1002/1529-0131(200009)43:9<1905::aid-anr1>3.0.co;2-p.
-
6.
Imani F, Rahimzadeh P. Interventional pain management according to evidence-based medicine. Anesth Pain Med. 2012;1(4):235-6. [PubMed ID: 24904805]. [PubMed Central ID: PMC4018708]. https://doi.org/10.5812/aapm.4514.
-
7.
Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy. 2017;33(3):659-6700. [PubMed ID: 28012636]. https://doi.org/10.1016/j.arthro.2016.09.024.
-
8.
Chen P, Huang L, Ma Y, Zhang D, Zhang X, Zhou J, et al. Intra-articular platelet-rich plasma injection for knee osteoarthritis: a summary of meta-analyses. J Orthop Surg Res. 2019;14(1):385. [PubMed ID: 31775816]. [PubMed Central ID: PMC6880602]. https://doi.org/10.1186/s13018-019-1363-y.
-
9.
Lana JF, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69-78. [PubMed ID: 28096631]. [PubMed Central ID: PMC5227106]. https://doi.org/10.46582/jsrm.1202011.
-
10.
Dengiz E, Özcan Ç, Güven Yİ, Uçar S, Ener BK, Sözen S, et al. Ozone gas applied through nebulization as adjuvant treatment for lung respiratory diseases due to COVID-19 infections: a prospective randomized trial. Med Gas Res. 2022;12(2):55-9. [PubMed ID: 34677153]. [PubMed Central ID: PMC8562398]. https://doi.org/10.4103/2045-9912.326001.
-
11.
Fernandez-Cuadros ME, Perez-Moro OS, Albaladejo-Florin MJ, Algarra-Lopez R. Ozone Decreases Biomarkers of Inflamation (C-Reactive Protein and Erytrocyte Sedimentation Rate) and Improves Pain, Function and Quality of Life in Knee Osteoarthrtitis Patients: A Before-and-After Study and Review of the Literature. Middle East J Rehabil Health. 2018;5(2). https://doi.org/10.5812/mejrh.64507.
-
12.
Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91-2. [PubMed ID: 21145753]. https://doi.org/10.1016/j.jcms.2010.11.001.
-
13.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-5. [PubMed ID: 20652370]. https://doi.org/10.1007/s10654-010-9491-z.
-
14.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. [PubMed ID: 22008217]. [PubMed Central ID: PMC3196245]. https://doi.org/10.1136/bmj.d5928.
-
15.
Babaei-Ghazani A, Najarzadeh S, Mansoori K, Forogh B, Madani SP, Ebadi S, et al. The effects of ultrasound-guided corticosteroid injection compared to oxygen-ozone (O(2)-O(3)) injection in patients with knee osteoarthritis: a randomized controlled trial. Clin Rheumatol. 2018;37(9):2517-27. [PubMed ID: 29796866]. https://doi.org/10.1007/s10067-018-4147-6.
-
16.
Raeissadat SA, Rayegani SM, Forogh B, Hassan Abadi P, Moridnia M, Rahimi Dehgolan S. Intra-articular ozone or hyaluronic acid injection: Which one is superior in patients with knee osteoarthritis? A 6-month randomized clinical trial. J Pain Res. 2018;11:111-7. [PubMed ID: 29379312]. [PubMed Central ID: PMC5757972]. https://doi.org/10.2147/jpr.s142755.
-
17.
Feng X, Beiping L. Therapeutic Efficacy of Ozone Injection into the Knee for the Osteoarthritis Patient along with Oral Celecoxib and Glucosamine. J Clin Diagn Res. 2017;11(9):Uc01-uc03. [PubMed ID: 29207809]. [PubMed Central ID: PMC5713831]. https://doi.org/10.7860/jcdr/2017/26065.10533.
-
18.
Lopes de Jesus CC, Dos Santos FC, de Jesus L, Monteiro I, Sant'Ana M, Trevisani VFM. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. PLoS One. 2017;12(7). e0179185. [PubMed ID: 28738079]. [PubMed Central ID: PMC5524330]. https://doi.org/10.1371/journal.pone.0179185.
-
19.
Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):485-92. [PubMed ID: 27056686]. https://doi.org/10.1007/s00167-016-4110-5.
-
20.
Invernizzi M, Stagno D, Carda S, Grana E, Picelli A, Smania N, et al. Safety of intra-articular oxygen-ozone therapy compared to intra-articular sodium hyaluronate in knee osteoarthritis: A randomized single blind pilot study. Int J Phys Med Rehabil. 2017;5(385):2.
-
21.
Ahmad K, Ali L, Maan MAM. The effects of injecting intra articular platelet-rich plasma on pain scores in knee joint osteoarthritis. Pafmj. 2021;71(4):1278-81. https://doi.org/10.51253/pafmj.v71i4.4791.
-
22.
Rahimzadeh P, Imani F, Faiz SHR, Entezary SR, Zamanabadi MN, Alebouyeh MR. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging. 2018;13:73-9. [PubMed ID: 29379278]. [PubMed Central ID: PMC5757490]. https://doi.org/10.2147/cia.s147757.
-
23.
Yu W, Xu P, Huang G, Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med. 2018;16(3):2119-25. [PubMed ID: 30186448]. [PubMed Central ID: PMC6122407]. https://doi.org/10.3892/etm.2018.6412.
-
24.
Rahimzadeh P, Imani F, Faiz SH, Alebouyeh MR, Azad-Ehyaei D, Bahari L, et al. Adding Intra-Articular Growth Hormone to Platelet Rich Plasma under Ultrasound Guidance in Knee Osteoarthritis: A Comparative Double-Blind Clinical Trial. Anesth Pain Med. 2016;6(6). e41719. [PubMed ID: 28975078]. [PubMed Central ID: PMC5560632]. https://doi.org/10.5812/aapm.41719.
-
25.
Abate M, Verna S, Schiavone C, Di Gregorio P, Salini V. Efficacy and safety profile of a compound composed of platelet-rich plasma and hyaluronic acid in the treatment for knee osteoarthritis (preliminary results). Eur J Orthop Surg Traumatol. 2015;25(8):1321-6. [PubMed ID: 26403468]. https://doi.org/10.1007/s00590-015-1693-3.
-
26.
Rahimzadeh P, Imani F, Faiz SH, Entezary SR, Nasiri AA, Ziaeefard M. Investigation the efficacy of intra-articular prolotherapy with erythropoietin and dextrose and intra-articular pulsed radiofrequency on pain level reduction and range of motion improvement in primary osteoarthritis of knee. J Res Med Sci. 2014;19(8):696-702. [PubMed ID: 25422652]. [PubMed Central ID: PMC4235087].
-
27.
Oo WM, Liu X, Hunter DJ. Pharmacodynamics, efficacy, safety and administration of intra-articular therapies for knee osteoarthritis. Expert Opin Drug Metab Toxicol. 2019;15(12):1021-32. [PubMed ID: 31709838]. https://doi.org/10.1080/17425255.2019.1691997.
-
28.
Huétink K, Stoel BC, Watt I, Kloppenburg M, Bloem JL, Malm SH, et al. Identification of factors associated with the development of knee osteoarthritis in a young to middle-aged cohort of patients with knee complaints. Clin Rheumatol. 2015;34(10):1769-79. [PubMed ID: 25213328]. https://doi.org/10.1007/s10067-014-2774-0.
-
29.
Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, et al. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am J Sports Med. 2019;47(2):347-54. [PubMed ID: 30545242]. https://doi.org/10.1177/0363546518814532.
-
30.
Charlesworth J, Fitzpatrick J, Perera NKP, Orchard J. Osteoarthritis- a systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet Disord. 2019;20(1):151. [PubMed ID: 30961569]. [PubMed Central ID: PMC6454763]. https://doi.org/10.1186/s12891-019-2525-0.
-
31.
Kanchanatawan W, Arirachakaran A, Chaijenkij K, Prasathaporn N, Boonard M, Piyapittayanun P, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1665-77. [PubMed ID: 26387122]. https://doi.org/10.1007/s00167-015-3784-4.
-
32.
Laudy AB, Bakker EW, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49(10):657-72. [PubMed ID: 25416198]. https://doi.org/10.1136/bjsports-2014-094036.
-
33.
Smelter E, Hochberg MC. New treatments for osteoarthritis. Curr Opin Rheumatol. 2013;25(3):310-6. [PubMed ID: 23425965]. https://doi.org/10.1097/BOR.0b013e32835f69b4.
-
34.
Simental-Mendía MA, Vílchez-Cavazos JF, Martínez-Rodríguez HG. [Platelet-rich plasma in knee osteoarthritis treatment]. Cir Cir. 2015;83(4):352-8. Spanish. [PubMed ID: 26116039]. https://doi.org/10.1016/j.circir.2014.06.001.
-
35.
Say F, Gürler D, Yener K, Bülbül M, Malkoc M. Platelet-rich plasma injection is more effective than hyaluronic acid in the treatment of knee osteoarthritis. Acta Chir Orthop Traumatol Cech. 2013;80(4):278-83. [PubMed ID: 24119476].
-
36.
Borrelli E, Alexandre A, Iliakis E, Alexandre A, Bocci V. Disc Herniation and Knee Arthritis as Chronic Oxidative Stress Diseases: The Therapeutic Role of Oxygen Ozone Therapy. J Arthritis. 2015;4(3). https://doi.org/10.4172/2167-7921.1000161.
-
37.
Raeissadat SA, Tabibian E, Rayegani SM, Rahimi-Dehgolan S, Babaei-Ghazani A. An investigation into the efficacy of intra-articular ozone (O(2)-O(3)) injection in patients with knee osteoarthritis: a systematic review and meta-analysis. J Pain Res. 2018;11:2537-50. [PubMed ID: 30498370]. [PubMed Central ID: PMC6207244]. https://doi.org/10.2147/jpr.s175441.
-
38.
Sconza C, Respizzi S, Virelli L, Vandenbulcke F, Iacono F, Kon E, et al. Oxygen–ozone therapy for the treatment of knee osteoarthritis: a systematic review of randomized controlled trials. Arthroscopy. 2020;36(1):277-86. [PubMed ID: 31679646]. https://doi.org/10.1016/j.arthro.2019.05.043.
-
39.
de Sire A, Stagno D, Minetto MA, Cisari C, Baricich A, Invernizzi M. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J Back Musculoskelet Rehabil. 2020;33(3):347-54. [PubMed ID: 32144974]. https://doi.org/10.3233/bmr-181294.
-
40.
Paolucci T, Agostini F, Bernetti A, Paoloni M, Mangone M, Santilli V, et al. Integration of focal vibration and intra-articular oxygen-ozone therapy in rehabilitation of painful knee osteoarthritis. J Int Med Res. 2021;49(2):300060520986705. [PubMed ID: 33641438]. [PubMed Central ID: PMC7923992]. https://doi.org/10.1177/0300060520986705.
-
41.
Hohmann E, Tetsworth K, Glatt V. Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur J Orthop Surg Traumatol. 2020;30(6):955-67. [PubMed ID: 32060630]. https://doi.org/10.1007/s00590-020-02623-4.
-
42.
Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2021;49(1):249-60. [PubMed ID: 32302218]. https://doi.org/10.1177/0363546520909397.
-
43.
Bennell KL, Hunter DJ, Paterson KL. Platelet-Rich Plasma for the Management of Hip and Knee Osteoarthritis. Curr Rheumatol Rep. 2017;19(5):24. [PubMed ID: 28386761]. https://doi.org/10.1007/s11926-017-0652-x.
-
44.
Gilat R, Haunschild ED, Knapik DM, Evuarherhe A, Parvaresh KC, Cole BJ. Hyaluronic acid and platelet-rich plasma for the management of knee osteoarthritis. Int Orthop. 2021;45(2):345-54. [PubMed ID: 32935198]. https://doi.org/10.1007/s00264-020-04801-9.
