Abstract
Context:
Robotic surgery is becoming the most common approach in minimally invasive urologic procedures. Robotic surgery offers less pain to patients because of smaller keyhole incisions and less tissue retraction and stretching of fascia and muscular fibers. Tailored pain regimens have also evolved and allowed patients to feel minimal to no discomfort after robotic urologic surgery, allowing in parallel better surgical outcomes. This study aims to analyze the most current pain regimens in robotic urologic surgery and to evaluate the most current pain protocols and corresponding outcomes.Evidence Acquisition:
A literature review was performed of published manuscripts utilizing Pubmed and Google Scholar on pain protocols for patients undergoing robotic urologic surgery.Results:
Multimodal analgesia is gaining ground in robotic urologic surgery. Regional analgesia includes four major modalities: Neuroaxial analgesia, intercostal blocks, tranvsersus abdominis plane blocks, and paravertebral blocks. Each approach has a different injection site, region of analgesia coverage, and duration of coverage depending upon local anesthesia and/or adjuvant utilized with advantages and disadvantages that make each modality unique and efficacious.Conclusions:
Robotic urologic surgery has offered the advantage of smaller incisions, faster recovery, less postoperative opioid consumption, and better surgical outcomes. Neuraxial, intercostal, transversus abdominis plane, and quadratus lumborum blocks are the best and most adopted approaches which offer optimal outcomes to patients.Keywords
Pain Robotic Urologic Surgery Urology Intercostal Tranvsersus Abdominis Plane Quadratus Lumborum Paravertebral Analgesia
1. Context
Robotic surgery is becoming the most commonly approach for postoperative analgesia in minimally invasive urologic procedures (1). Traditional open surgery exposed patients to significant doses of pain medication in the past, especially in the immediate post-operative setting. As a result, patients experienced increased morbidity such as prolonged ileus and increased hospital length of stay, as well as increased mortality (2). Over the past two decades, robotic-assisted laparoscopic urologic surgery has offered a faster recovery and less postoperative opioid consumption to patients. In this regard, advances in regional anesthesia have allowed patients to be discharged home typically on the same day (3, 4). Robotic surgery offers less discomfort to patients because of smaller keyhole incisions (e.g., small skin cuts for trocar insertion) and less tissue retraction and stretching of fascia and muscular fibers (5).
Tailored pain regimens have also evolved and allowed patients to feel minimal discomfort after robotic urologic surgery, allowing in parallel better surgical outcomes (6). At the 2019 American Society of Clinical Oncology (ASCO), Talwar et al. presented the results of a pain management program for patients undergoing robotic urologic surgeries, specifically robotic radical prostatectomies, robotic radical nephrectomies, and robotic partial nephrectomies [cited in (6)]. The vast majority of patients (68%) were discharged home without prescriptions for opioids, attesting to effective optimized intra-operative pain regimens and effective tailored regional analgesia for the immediate post-operative setting (6). Pain stimuli in robotic urologic surgery are attributed to multiple factors, including abdominal distention during pneumoperitoneum (e.g., carbon dioxide insufflation), which may lead to peritoneal irritation, referred pain related to diaphragmatic stretching, and finally, trocar incisional pain or pain at the abdominal wall extraction site (7). Thus, understanding fundamental pain pathways is requited to offer patients adequate local, regional, and/or systemic analgesia. The advances in recent years of ultrasound guided regional anesthesia blocks and longer lasting local anesthetics have been significant in developing strategies for effective pain management in these patients. Therefore, the present study aims to analyze the most common pain regimens in robotic urologic surgery and to evaluate the most current protocols and corresponding outcomes.
2. Evidence Acquisition
The present investigation involved a literature review of all published manuscripts on pain protocols for patients undergoing robotic urologic surgery and also related topics. The databases searched included PubMed and Google Scholar, and 57 published manuscripts were identified and reviewed.
3. Results
Pain regimens in robotic urologic surgery are flourishing. Urologic surgeons commonly participate with anesthesiologists, and other stakeholders such as hospital administration and nursing to optimize these multimodal approaches (8). Lowering pneumoperitoneum (traditionally at 15 mmHg) to reduce pressures has demonstrated postoperative reductions in abdominal pain, less ileus, and decreased opioid consumption (9). Patient positioning and appropriate padding of all pressure points are crucial and have been shown to decrease opioid consumption (10). Pre- emptive analgesia, which is administered before surgical incision, prevents central sensitization, commonly resulting from local inflammation, and provides better pain control (11). Multimodal analgesia strategies have also expanded in recent years in particular with adjuvant medications and ultrasound guided nerve blocks (12-14). This concept is based on utilizing different groups of pain medications for additive and/or synergistic effects and results in reduction of side effects of larger doses of each medication seen for example with administration of only large amounts of opioids (7, 15). Multiple adjuvant drugs have been identified through clinical studies, including ketamine, NSAIDs, gabapentin, paracetamol, intravenous lidocaine, and magnesium (16-19). Adjuvants medications that provide enhanced local anesthetic duration of activity include dexamethasone, alpha-2 agonists such as dexmedetomidine and clonidine, NMDA antagonists such as ketamine, neostigmine, epinephrine, and sodium bicarbonate (20-24).
Regional analgesia in robotic urologic surgery includes four major modalities: neuroaxial, intercostal, transversus abdominis plane (TAP), and paravertebral (PVB) blocks and anatomic considerations are described:
3.1. Neuraxial Blockade
Previous studies have included minimally invasive colorectal procedures which have used epidural anesthesia as a pain regimen; however, there has been limited recent application in urologic surgery (25). It was found that this approach is not recommended in robotic urologic surgery because of potential serious side effects that include hypotension, pruritus, and respiratory depression (7). Koning et al. studied the effects of neuraxial combination of morphine and bupivacaine on recovery state in robot-assisted radical prostatectomy (26). The authors had 155 patients randomly administered intrathecal 12.5 mg bupivacaine/300 micrograms of morphine. They demonstrated that intrathecal bupivacaine/morphine was an efficient and safe option for robot-assisted radical prostatectomy.
3.2. Intercostal Block (IC)
The anterior branches of T7 innervate the anterior abdominal wall to L1 (7). Blocking the T7 - T11 intercostal nerves has been previously described in laparoscopic upper abdominal surgery and can be extrapolated to robotic urologic surgery.
3.3. Transversus Abdominis Plane Block (TAP)
If considering a laparoscopic or robotic procedure involving trocar insertion on both sides of the abdomen (e.g., right and left), blocking intercostal nerves 9 to 11 is beneficial for pain control (27). In addition to administering TAP blocks directly, they may be provided either with ultrasound guidance or laparoscopic guidance (28-30). Covotta et al. studied in their prospective randomized clinical trial the consequences of TAP block under ultrasound guidance on both chronic and acute postoperative pain following robotic partial nephrectomy (31). They evaluated 96 patients who underwent a robotic partial nephrectomy. Their primary endpoint was opioid utilization on the 1st postoperative day. Other subsidiary outcomes included nausea and vomiting after the surgery in the acute setting and chronic pain. This study concluded that TAP blocks significantly reduced somatic pain and morphine consumption (but no effect on visceral pain) and decreased the occurrence of chronic pain (31).
3.4. Paravertebral Block (PVB)
Previous reports have evaluated effectiveness of this technique during laparoscopic nephrectomy, and these studies have found that PVB decreases pain levels and decreases the amount of systemic opioid administration (32). In this regard, Herling et al. attempted to compare effectiveness of total intravenous anesthesia with inhalational anesthesia for adults undergoing transabdominal robotic-assisted laparoscopic surgery (33). The authors found no statistical difference between the two approaches when they compared propofol with inhaled sevoflurane and desflurane in terms of postoperative pain level.
The role of each of these blocks clinically and studies highlighted them are described:
3.4.1. Neuraxial Blockade
Neuraxial blockades are utilized in surgery, obstetrics, and chronic pain, most commonly involving the lower abdomen and/or lower extremities. Techniques typically revolve around injection of anesthesia into the epidural space or the subarachnoid space to provide epidural and spinal anesthesia.
Anesthesia techniques for many urological procedures also can include caudal epidural techniques in particular in the pediatric population (34). In general, clinicians should use small- diameter needles to limit the risk of postdural puncture headache whenever possible (35). A midline or paramedian approach is taken for neuraxial blocks. The method chosen is predominantly a function of patient characteristics (e.g., body habitus, spine abnormalities, comorbidities), skill/preference of the clinician, and involved sensory levels. Midline approaches allow for a more direct, predictable path. Still, they require a relatively greater amount of spinal flexion, limiting its utility in some patients (e.g., those with severe scoliosis). Once an approach is decided, the needle insertion site can be determined. Palpation has traditionally been used to identify anatomical landmarks that facilitate proper insertion; however, ultrasound guidance is becoming more frequently used to directly visualize intervertebral spaces and the associated needle insertion point, depth, and angle in real-time preprocedural (36). While some studies have shown superior accuracy or ease of needle placement assisted by newer techniques involving ultrasound, it remains controversial as others have shown no clear advantage (37, 38).
With regards to the literature, one study conducted by Jiang et al. showed increased first- pass success rates in patients with greater predicted puncture difficulty but not in patients who were easily punctured (39). Both techniques involve identifying the desired intervertebral space, numbing the area in which the needle will be inserted with local anesthesia, and advancing the needle through the skin, soft tissue, and spinal ligaments until the epidural space (epidural block) or subarachnoid space (spinal block) is reached. Epidural anesthesia also involves catheter placement for additional medication if desired, whereas this is not required for spinal anesthesia, which typically is only used for a single dose of analgesia. The dose of anesthesia is dependent on the drug of choice and the degree of blockade needed.
3.4.2. Intercostal Blockade
Intercostal nerve blocks can be used for thoracic procedures and injuries such as thoracotomy, chest tube placement, rib fracture, post-operative lumpectomy pain control, and upper abdominal procedures (40). Similar to neuraxial block, anatomic landmarks alone or with ultrasound assistance provide valuable guidance. Ultrasound assistance is more clearly superior to landmark-alone methods in intercostal nerve blocks based on our literature review (41).
The patient should be positioned appropriately (i.e., sitting, lateral, or prone), and the desired rib should be palpated at the mid-posterior axillary line. The transverse spinal process should be identified with the transducer positioned medially when ultrasound is used. The device's subsequent lateral movement should take place until a proximal view of the intercostal space is obtained with shared visualization of the ribs, intercostal muscles, and pleura. Local analgesia administration to the needle infiltration site and a needle can then be inserted with subsequent cephalad advancement to the inferior edge of the rib and into the subcostal groove for full anesthesia administration.
Another regional block technique used for postoperative pain is a TAP block (28-30). This block involves ultrasound-guided needle insertion into the inter-fascial plane between muscles of the abdomen followed by the release of local anesthetic. Often, TAP blocks are performed during or near the conclusion of the urogenital procedure. TAP blocks can be unilateral or bilateral, depending upon the procedure. As denoted by the name, unilateral blocks are performed on procedures involving one side of the body, such as appendectomies, cholecystectomies, nephrectomies, and renal transplants. Bilateral blocks are used if the procedure involves the midline or transverse abdominis; these include certain hernial repairs, radical retropubic prostatectomy, and laparoscopic surgeries. TAP blocks are preferred for analgesia over opioid use or epidural anesthesia for abdominal surgeries because they are simpler to place and lower risk. Complications from TAP blocks are also rare due to the high vascularization of the area, another benefit of this type of block (42). However, successful pain management by the TAP block depends upon the area of coverage of local anesthetic across the inter-fascial plane. Thus, an adequate local anesthetic volume and the procedure's technical aspects should be chosen and performed carefully for maximum anesthetic effect.
A needle is guided via ultrasound between muscles of the abdomen, most commonly the transversus abdominis and internal oblique. Two 20 mL syringes are prepared with a local anesthetic such as ropivacaine, bupivacaine, or liposomal bupivacaine. These volumes are considered sufficiently high volume enough for the adequate spread of the anesthetic (43). For procedures conducted with a conscious patient, one 5 mL syringe filled with lidocaine should also be prepared to anesthetize the skin. It has been suggested that optimal dosages for transversus abdominis plane blocks are not well defined (43). The inter-fascial plane of the transversus abdominis and the internal oblique can be targeted in subcostal, lateral, or posterior. These approaches target varying thoracic dermatomes, and the appropriate approach is chosen depending on the procedure being performed.
For open and laparoscopic cholecystectomies, a subcostal approach is indicated. The subcostal approach targets the anterior abdominal wall and releases local anesthetic between the transverse abdominis muscle and posterior rectus sheath, thus blocking T6 - T9 dermatomes. The needle should enter above the rectus abdominis muscle until it reaches the desired location. A high-frequency ultrasound probe is positioned between the xiphoid process and the anterosuperior iliac spine at the anterior axillary line to assist needle placement. One study demonstrated positive postoperative outcomes using continuous infusion of 0.25% bupivacaine (44).
The lateral approach is mostly used as it is the preferred approach for most abdominal procedures, including but not limited to laparoscopic surgeries, hernia repairs, and radical retropubic prostatectomies. This approach involves an injection of local anesthetic between the transversus abdominis and internal oblique muscles to target T10 - T12 dermatomes. A high- frequency ultrasonographic transducer is placed among the iliac crest and subcostal border and advanced to the transversus abdominis plane. When all three layers of muscle in the abdominal wall are visualized, the local anesthetic should be deposited, ideally reaching the plane between transversus abdominis and internal oblique muscles. It should be noted that some suggest a lateral approach as less preferable to the subcostal or posterior approach due to less favorable postoperative pain, and thus suggest consideration for lateral combined with subcostal approach (43).
A third approach is a posterior approach, which is used primarily for renal procedures, including nephrectomies and transplants. The posterior approach involves the identification of the lumbar triangle of Petit or the quadratus lumborum muscle. At this point, a high-frequency ultrasound probe is first inserted in the midaxillary line, then advanced laterally and posteriorly. The local anesthetic should be injected between the internal oblique and transversus abdominis muscle similar to the lateral approach, but this time more posteriorly to target T9 - T12 dermatomes. It has been suggested that the posterior approach leads to less postoperative pain than the widely used lateral approach for lower abdominal procedures (45).
3.4.3. Ultrasound-Guided Quadratus Lumborum Block
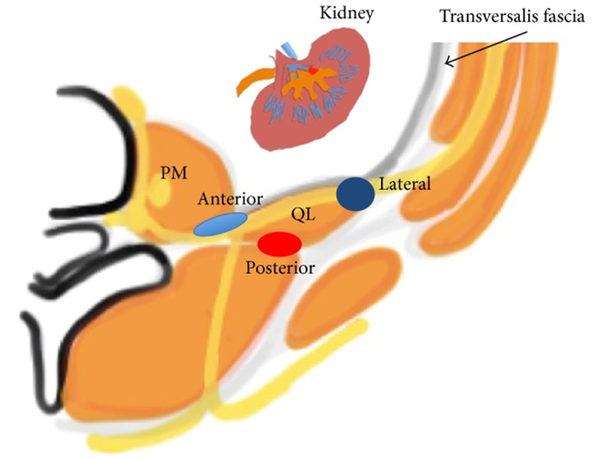
The quadratus lumborum (QL) block is a newer regional technique involving the delivery of anesthesia to the intercostal nerves of the abdominal wall. The QL block can be used in abdominopelvic cases where a broad abdomen coverage is required. Examples include colorectal surgery, C-section, nephrolithotomy, nephrectomy, and gynecological procedures (46-48). It is also useful in robotic cases where multiple port sites are scattered over various quadrants (49). Because the QL block usually gets T12 - L1 branches, people have also used QL blocks for hip surgeries (50, 51). This block can reduce pain scores and post-operative opioid consumption (52, 53) QL muscle stretches from the iliac crest to the 12th rib with medial attachments to the lumbar transverse processes. It lies anterior to the erector spinae muscle and posterior to the psoas major (PM). The ventral rami of the lower thoracic nerves run along the anterior surface of the QL before jumping into the TAP plane. A local anesthetic (LA) placed in the potential space between the QL and PM will anesthetize the nerves supplying the abdominal wall.
The QL block, first described by Blanco, can be subdivided based on an anatomical approach (54, 55). In the QL1 approach, the needle is guided anterolateral to the QL muscle. In the QL II, the needle is advanced posterior to the QL between the QL and latissimus dorsi (LD). A newer approach termed QL3 or “trans muscular/TQL” approach has gained popularity (56, 57). In this approach, the needle is advanced through the erector spinae (ES) muscle and placed anterior to the QL posterior to the PM. The goal of the QL block is for the LA to travel in the cephalad direction and enter the paravertebral space of the lower thorax by the lumbodorsal arch, thus providing adequate analgesia of the abdomen wall. In addition, The QL blocks the upper reaches of the abdomen and contributes to visceral analgesia following low thoracic sympathetic block (reference on “visceral analgesia”).
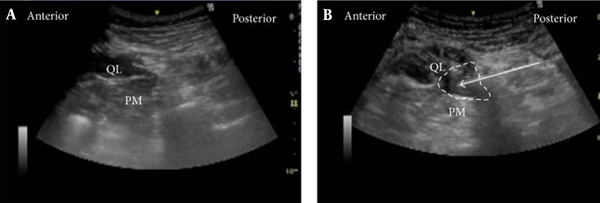
The original technique described by Blanco is as follows: Using a Curvilinear probe with an image depth of 1 - 9 cm. The probe is located over the anterosuperior iliac spine and propelled to the cranial side until the all abdominal wall muscle layers were recognized.
The external oblique muscle was continued to the posterior and lateral direction until its posterior margin was visualized (hook sign), leaving below the internal oblique muscle, similar a cover over the quadratus lumborum muscle. The transducer was moved down to detect a lucent hyperechoic track that indicted with the middle layer of the thoracolumbar fascia. The sonovisible needle by in-plane approach was conducted from medial (anterior) to lateral (posterior). The best point of injection was defined using hydrodissection. The expansion of local anesthetic was posterior to medial rather than anterior to lateral direction (54).
Out of 12 studies on QLB in urologic/abdominal surgery reviewed, only one study investigated the effect of QLB in robotic-assisted urological surgery. Multiple studies highlight the possible benefits of QLB in open and laparoscopic abdominal surgery. Given these promising results, future studies should be conducted to assess the specific indications, benefits, and risks of QLB in robotic urological surgeries such as radical prostatectomy and radical and partial nephrectomies.
A 2019 randomized control trial compared QLB vs TAP block in laparoscopic colorectal surgery. Compared to TAP block, the study found that patients who underwent QLB used significantly fewer opioids in the post-operative period within the first 24 - 48 hours (52).
A 2021 randomized control double-blind study investigated the effectiveness of the QL block for robotic-assisted partial nephrectomy (RAPN). Compared to placebo injection, the study
found that patients who underwent QL block reported decreased pain scores and consumed fewer opioids in the post-op period (53). See Figures 1 and 2.
Anatomical view of the QL block (lateral, anterior, and posterior) (50)
Ultrasound Images of anterior QLB. A, pre-injection; B, post-injection (50).
4. Discussion
The present investigation aimed to analyze currently available pain regimens in robotic surgeries to evaluate the most updated protocols and corresponding outcomes. We conducted a literature review comparing the technique and outcomes of the neuraxial block, intercostal block, transversus abdominis plane block, and quadratus lumborum block in robotic surgeries (Table 1).
Technique Prons and Cons Summary
| Technique | LA Injection Site | Analgesia Coverage | Pros | Cons |
|---|---|---|---|---|
| Neuraxial block | Intrathecal space or epidural space | Spinal cord below T10 a | Provide true analgesia, motor, sensory and autonomic (sympathetic) blockade | Hypotension, decreased cardia output, urinary retention, PDPH |
| Intercostal block | Subcostal groove | Ipsilateral sensory and motor fibers of the intercostal nerves | Technique is simple and can be done in various anatomical locations | No visceral abdominal analgesia. Risk of pneumothorax |
| Transvers abdominis plane block | Between the internal oblique muscle and transvers abdominis muscle | Nerves supplying anterior abdominal wall (T6 - L4) | Technique is simple and can be done in various anatomical locations | No visceral abdominal analgesia. |
| Quadratus lumborum block | Between quadratus lumborum and psoas major b | Abdominal wall (T12 - L1) | Achieve both somatic and visceral analgesia | Difficulty of technique |
As a primary anesthetic, neuraxial blocks have been proven most useful in lower abdominal, inguinal, urological, rectal, and lower extremity surgery. Out of the different nerve blocks evaluated in this study, a neuraxial blockade is the most widely studied and performed worldwide. The primary benefit of this technique is the achievement of both visceral and somatic pain response, which provides excellent operating conditions for surgeries below the umbilicus. In addition, most Anesthesiologists have vast experience performing neuraxial blockade. Its technique has been well established for decades, and expensive ultrasound machines are unnecessary.
Neuraxial anesthesia is an excellent choice for urologic and robotic surgeries but can still be associated with various complications. These adverse effects include post-dural puncture headache, decreased blood pressure and cardiac output, and patient anxiety due to loss of lower extremity motor function. Neuraxial block also carries an increased list of contraindications compared to other, more superficial nerve blocks. These common contraindications include coagulation abnormalities and hypovolemia and should be used with caution in patients on blood thinners due to the risk of epidural hematomas.
The intercostal nerve (IC) block has provided adequate analgesia in upper abdominal laparoscopic surgery and robotic-assisted thoracic surgery. Compared to neuraxial block, this type of regional technique is used more frequently as an adjunct to a primary analgesic technique to reduce postop pain. The primary benefit of this technique is the ability to provide specific dermatomal anterior abdominal wall analgesia. This technique can significantly reduce bodily pain caused by the robotic arm ports. Given the anatomical location, it is principal to define the risk of pneumothorax. The major concern with this technique is that it does not block visceral abdominal pain and has limited analgesia below the umbilicus. Although its use in robotic urologic surgery has not been well described, the benefit of this block as an adjunct to a perioperative pain regimen is worth exploring.
The TAP block is another blockade technique used as an adjunct in perioperative pain regimens. Like the intercostal block, it is usually done at the end of a procedure to provide postop analgesia and reduce opioid consumption. This technique also shares the benefit of simplicity and decreased risk compared to neuraxial blockade. This block's versatility allows the anesthesiologist to choose different anatomical approaches depending on the surgery being performed. For nephrectomies, a posterior approach is recommended, while a lateral approach provides better analgesia for prostatectomy. Although associated with minimal risks, the primary disadvantage of TAP blocks is they are limited to somatic analgesia, and their efficacy depends on local anesthetic infiltration of the interfacial plane.
The QL block offers a unique advantage to both the TAP and intercostal blocks. Given the anatomical proximity of paravertebral space to the fascial plane between QL muscle and the PM, adequate infiltration of a local anesthetic to this plane can lead to a paravertebral block providing both somatic and visceral abdominal wall analgesia. QL blocks have been used in the perioperative period in robotic urologic nephrectomies with good outcomes. Unfortunately, the use of this block in other urologic surgeries has not been studied. The ability to perform a QL block that successfully travels in a cephalad direction to the paravertebral space depends on the anesthesiologist's technique and expertise. Given that it is a relatively new technique, a limited number of providers are confident in mastering this block.
All these techniques carry a similar risk in patients with platelet dysfunction or those who take blood thinners. Compared to the neuraxial block, the intercostal block, TAP block, and quadratus lumborum block offer significantly less invasive methods of achieving adequate analgesia in abdominal surgeries. With the rise of ultrasound-guided nerve blocks, it is important to study different techniques that can be applied to achieve adequate analgesia while at the same time decreasing morbidity and mortality in the patient population. The availability of different regional blocks in robotic surgeries increases the opportunity for the anesthesiologist to provide patient-specific pain regimens and improve overall outcomes. We encourage future studies to explore the safety and efficacy of these different techniques in robotic urological surgeries.
4.1. Conclusions
Minimally invasive surgery and robotic surgery have offered the advantage of smaller incisions, faster recovery, less pain medication, reduced incidence of post-operative ileus, and better surgical outcomes. Of the many regional techniques described in the literature, neuraxial, intercostal, TAP, and QL blocks are the best approaches which offer optimal outcomes to patients underdoing robotic urologic surgery. In all cases these techniques reduce opioid consumption and reduce hospital stays for urological procedures. Adjuvant medications can prolong local anesthetic blockade and reduce postoperative opioid consumption. In summary, an appropriate pain regimen tailored to each patient utilizing newer regional anesthesia techniques and adjuvant medications likely will allow for reduced hospital stays, fewer nosocomial infections, and also less opioid consumption as well as the potential for reduced hospital costs in urological surgery. More studies are warranted to best define the role for each of these techniques and adjuvant medication in order to determine best practice for pain management in urological surgery.
References
-
1.
Sheetz KH, Claflin J, Dimick JB. Trends in the Adoption of Robotic Surgery for Common Surgical Procedures. JAMA Netw Open. 2020;3(1). e1918911. [PubMed ID: 31922557]. [PubMed Central ID: PMC6991252]. https://doi.org/10.1001/jamanetworkopen.2019.18911.
-
2.
Tevis SE, Kennedy GD. Postoperative complications and implications on patient-centered outcomes. J Surg Res. 2013;181(1):106-13. [PubMed ID: 23465392]. [PubMed Central ID: PMC3637983]. https://doi.org/10.1016/j.jss.2013.01.032.
-
3.
Ploussard G, Almeras C, Beauval JB, Gautier JR, Loison G, Salin A, et al. Same-day discharge surgery for robot-assisted radical prostatectomy in the era of ERAS and prehabilitation pathways: a contemporary, comparative, feasibility study. World J Urol. 2022;40(6):1359-65. [PubMed ID: 32065277]. https://doi.org/10.1007/s00345-020-03119-w.
-
4.
Abaza R, Murphy C, Bsatee A, Brown DJ, Martinez O. Single-port Robotic Surgery Allows Same-day Discharge in Majority of Cases. Urology. 2021;148:159-65. [PubMed ID: 33217457]. https://doi.org/10.1016/j.urology.2020.08.092.
-
5.
Midgley S, Tolley DA. Anaesthesia for laparoscopic surgery in urology. EAU-EBU Update Series. 2006;4(6):241-5. https://doi.org/10.1016/j.eeus.2006.08.003.
-
6.
The ASCO Post. 2019 ASCO: Pain Management Program for Patients Undergoing Robotic Urologic Surgery. New York, USA: The ASCO Post; 2019, [cited 8 Dec 2021]. Available from: https://ascopost.com/News/60118.
-
7.
Batley SE, Prasad V, Vasdev N, Mohan SG. Post-Operative Pain Management in Patients Undergoing Robotic Urological Surgery. Curr Urol. 2016;9(1):5-11. [PubMed ID: 26989364]. [PubMed Central ID: PMC4789944]. https://doi.org/10.1159/000442843.
-
8.
Efstathiou G, Batistaki C, Soulioti E, Roungeris L, Matsota P. Opioid-Free Anesthesia and Postoperative Cognitive Dysfunction After Minor Urological Surgery: A Case Series Study. Anesth Pain Med. 2022;12(1). e122094. [PubMed ID: 35433375]. [PubMed Central ID: PMC8995876]. https://doi.org/10.5812/aapm.122094.
-
9.
Yasir M, Mehta KS, Banday VH, Aiman A, Masood I, Iqbal B. Evaluation of post operative shoulder tip pain in low pressure versus standard pressure pneumoperitoneum during laparoscopic cholecystectomy. Surgeon. 2012;10(2):71-4. [PubMed ID: 22385527]. https://doi.org/10.1016/j.surge.2011.02.003.
-
10.
Walls J. Patient Positioning During Anesthesia: Robotic Surgery and Other Advanced Technology. New York, USA: Clinical Pain Advisor; 2019, [cited 20 Jul 2020]. Available from: https://www.clinicalpainadvisor.com/decision-support-in-medicine/anesthesiology/patient-positioning-during-anesthesia-robotic-surgery-and-other-advanced-technology/.
-
11.
Breda A, Bui MH, Liao JC, Schulam PG. Association of bowel rest and ketorolac analgesia with short hospital stay after laparoscopic donor nephrectomy. Urology. 2007;69(5):828-31. [PubMed ID: 17482915]. https://doi.org/10.1016/j.urology.2007.01.083.
-
12.
Edinoff AN, Girma B, Trettin KA, Horton CC, Kaye AJ, Cornett EM, et al. Novel Regional Nerve Blocks in Clinical Practice: Evolving Techniques for Pain Management. Anesth Pain Med. 2021;11(4). e118278. [PubMed ID: 34692446]. [PubMed Central ID: PMC8520672]. https://doi.org/10.5812/aapm.118278.
-
13.
Edinoff AN, Houk GM, Patil S, Bangalore Siddaiah H, Kaye AJ, Iyengar PS, et al. Adjuvant Drugs for Peripheral Nerve Blocks: The Role of Alpha-2 Agonists, Dexamethasone, Midazolam, and Non-steroidal Anti-inflammatory Drugs. Anesth Pain Med. 2021;11(3). e117197. [PubMed ID: 34540647]. [PubMed Central ID: PMC8438706]. https://doi.org/10.5812/aapm.117197.
-
14.
Edinoff AN, Fitz-Gerald JS, Holland KAA, Reed JG, Murnane SE, Minter SG, et al. Adjuvant Drugs for Peripheral Nerve Blocks: The Role of NMDA Antagonists, Neostigmine, Epinephrine, and Sodium Bicarbonate. Anesth Pain Med. 2021;11(3). e117146. [PubMed ID: 34540646]. [PubMed Central ID: PMC8438710]. https://doi.org/10.5812/aapm.117146.
-
15.
Malik KM, Imani F, Beckerly R, Chovatiya R. Risk of Opioid Use Disorder from Exposure to Opioids in the Perioperative Period: A Systematic Review. Anesth Pain Med. 2020;10(1). e101339. [PubMed ID: 32337175]. [PubMed Central ID: PMC7158240]. https://doi.org/10.5812/aapm.101339.
-
16.
Imani F, Varrassi G. Ketamine as Adjuvant for Acute Pain Management. Anesth Pain Med. 2019;9(6). e100178. [PubMed ID: 32280623]. [PubMed Central ID: PMC7119219]. https://doi.org/10.5812/aapm.100178.
-
17.
Tully J, Jung JW, Patel A, Tukan A, Kandula S, Doan A, et al. Utilization of Intravenous Lidocaine Infusion for the Treatment of Refractory Chronic Pain. Anesth Pain Med. 2020;10(6). e112290. [PubMed ID: 34150583]. [PubMed Central ID: PMC8207879]. https://doi.org/10.5812/aapm.112290.
-
18.
Urits I, Jung JW, Amgalan A, Fortier L, Anya A, Wesp B, et al. Utilization of Magnesium for the Treatment of Chronic Pain. Anesth Pain Med. 2021;11(1). e112348. [PubMed ID: 34221945]. [PubMed Central ID: PMC8236839]. https://doi.org/10.5812/aapm.112348.
-
19.
Soleimanpour H, Imani F, Dolati S, Soleimanpour M, Shahsavarinia K. Management of pain using magnesium sulphate: a narrative review. Postgrad Med. 2022;134(3):260-6. [PubMed ID: 35086408]. https://doi.org/10.1080/00325481.2022.2035092.
-
20.
Imani F, Zaman B, De Negri P. Postoperative Pain Management: Role of Dexmedetomidine as an Adjuvant. Anesth Pain Med. 2020;10(6). e112176. [PubMed ID: 34150582]. [PubMed Central ID: PMC8207883]. https://doi.org/10.5812/aapm.112176.
-
21.
Mahmoudi K, Rashidi M, Soltani F, Savaie M, Hedayati E, Rashidi P. Comparison of Intercostal Nerve Block with Ropivacaine and Ropivacaine-Dexmedetomidine for Postoperative Pain Control in Patients Undergoing Thoracotomy: A Randomized Clinical Trial. Anesth Pain Med. 2021;11(6). e118667. [PubMed ID: 35291405]. [PubMed Central ID: PMC8908443]. https://doi.org/10.5812/aapm.118667.
-
22.
Alimian M, Imani F, Rahimzadeh P, Faiz SHR, Bahari-Sejahrood L, C. Hertling A. Adding Dexmedetomidine to Bupivacaine in Ultrasound-guided Thoracic Paravertebral Block for Pain Management after Upper Abdominal Surgery: A Double-blind Randomized Controlled Trial. Anesth Pain Med. 2021;11(6). e120787. [PubMed ID: 35291399]. [PubMed Central ID: PMC8908442]. https://doi.org/10.5812/aapm.120787.
-
23.
Imani F, Farahmand Rad R, Salehi R, Alimian M, Mirbolook Jalali Z, Mansouri A, et al. Evaluation of Adding Dexmedetomidine to Ropivacaine in Pediatric Caudal Epidural Block: A Randomized, Double-blinded Clinical Trial. Anesth Pain Med. 2021;11(1). e112880. [PubMed ID: 34221950]. [PubMed Central ID: PMC8241816]. https://doi.org/10.5812/aapm.112880.
-
24.
Margulis R, Francis J, Tischenkel B, Bromberg A, Pedulla D, Grtisenko K, et al. Comparison of Dexmedetomidine and Dexamethasone as Adjuvants to Ultra-Sound Guided Interscalene Block in Arthroscopic Shoulder Surgery: A Double-Blinded Randomized Placebo-Controlled Study. Anesth Pain Med. 2021;11(3). e117020. [PubMed ID: 34540645]. [PubMed Central ID: PMC8438728]. https://doi.org/10.5812/aapm.117020.
-
25.
Joshi GP, Bonnet F, Kehlet H, Prospect collaboration. Evidence-based postoperative pain management after laparoscopic colorectal surgery. Colorectal Dis. 2013;15(2):146-55. [PubMed ID: 23350836]. https://doi.org/10.1111/j.1463-1318.2012.03062.x.
-
26.
Koning MV, de Vlieger R, Teunissen AJW, Gan M, Ruijgrok EJ, de Graaff JC, et al. The effect of intrathecal bupivacaine/morphine on quality of recovery in robot-assisted radical prostatectomy: a randomised controlled trial. Anaesthesia. 2020;75(5):599-608. [PubMed ID: 31845316]. [PubMed Central ID: PMC7187216]. https://doi.org/10.1111/anae.14922.
-
27.
Pourseidi B, Khorram-Manesh A. Effect of intercostals neural blockade with Marcaine (bupivacaine) on postoperative pain after laparoscopic cholecystectomy. Surg Endosc. 2007;21(9):1557-9. [PubMed ID: 17342558]. https://doi.org/10.1007/s00464-006-9181-9.
-
28.
Imani F, Rahimzadeh P, Faiz HR, Abdullahzadeh-Baghaei A. An Evaluation of the Adding Magnesium Sulfate to Ropivacaine on Ultrasound-Guided Transverse Abdominis Plane Block After Abdominal Hysterectomy. Anesth Pain Med. 2018;8(4). e74124. [PubMed ID: 30250819]. [PubMed Central ID: PMC6139531]. https://doi.org/10.5812/aapm.74124.
-
29.
Faiz SHR, Alebouyeh MR, Derakhshan P, Imani F, Rahimzadeh P, Ghaderi Ashtiani M. Comparison of ultrasound-guided posterior transversus abdominis plane block and lateral transversus abdominis plane block for postoperative pain management in patients undergoing cesarean section: a randomized double-blind clinical trial study. J Pain Res. 2018;11:5-9. [PubMed ID: 29296094]. [PubMed Central ID: PMC5741073]. https://doi.org/10.2147/JPR.S146970.
-
30.
Gharaei H, Imani F, Almasi F, Solimani M. The Effect of Ultrasound-guided TAPB on Pain Management after Total Abdominal Hysterectomy. Korean J Pain. 2013;26(4):374-8. [PubMed ID: 24156004]. [PubMed Central ID: PMC3800710]. https://doi.org/10.3344/kjp.2013.26.4.374.
-
31.
Covotta M, Claroni C, Costantini M, Torregiani G, Pelagalli L, Zinilli A, et al. The Effects of Ultrasound-Guided Transversus Abdominis Plane Block on Acute and Chronic Postsurgical Pain After Robotic Partial Nephrectomy: A Prospective Randomized Clinical Trial. Pain Med. 2020;21(2):378-86. [PubMed ID: 31504875]. https://doi.org/10.1093/pm/pnz214.
-
32.
Clendenen SR, Wehle MJ, Rodriguez GA, Greengrass RA. Paravertebral block provides significant opioid sparing after hand-assisted laparoscopic nephrectomy: an expanded case report of 30 patients. J Endourol. 2009;23(12):1979-83. [PubMed ID: 19919257]. https://doi.org/10.1089/end.2009.0095.
-
33.
Herling SF, Dreijer B, Wrist Lam G, Thomsen T, Moller AM. Total intravenous anaesthesia versus inhalational anaesthesia for adults undergoing transabdominal robotic assisted laparoscopic surgery. Cochrane Database Syst Rev. 2017;4. CD011387. [PubMed ID: 28374886]. [PubMed Central ID: PMC6478279]. https://doi.org/10.1002/14651858.CD011387.pub2.
-
34.
Farahmand Rad R, Imani F, Emami A, Salehi R, Ghavamy AR, Shariat AN. Postoperative Pain Management: Efficacy of Caudal Tramadol in Pediatric Lower Abdominal Surgery: A Randomized Clinical Study. Anesth Pain Med. 2021;11(4). e119346. [PubMed ID: 34692449]. [PubMed Central ID: PMC8520683]. https://doi.org/10.5812/aapm.119346.
-
35.
Tsen LC, Hepner DL. Needles used for spinal anesthesia. Expert Rev Med Devices. 2006;3(4):499-508. [PubMed ID: 16866646]. https://doi.org/10.1586/17434440.3.4.499.
-
36.
Yoo S, Kim Y, Park SK, Ji SH, Kim JT. Ultrasonography for lumbar neuraxial block. Anesth Pain Med (Seoul). 2020;15(4):397-408. [PubMed ID: 33329842]. [PubMed Central ID: PMC7724125]. https://doi.org/10.17085/apm.20065.
-
37.
Young B, Onwochei D, Desai N. Conventional landmark palpation vs. preprocedural ultrasound for neuraxial analgesia and anaesthesia in obstetrics - a systematic review and meta-analysis with trial sequential analyses. Anaesthesia. 2021;76(6):818-31. [PubMed ID: 32981051]. https://doi.org/10.1111/anae.15255.
-
38.
Arzola C, Mikhael R, Margarido C, Carvalho JC. Spinal ultrasound versus palpation for epidural catheter insertion in labour: A randomised controlled trial. Eur J Anaesthesiol. 2015;32(7):499-505. [PubMed ID: 25036283]. https://doi.org/10.1097/EJA.0000000000000119.
-
39.
Jiang L, Zhang F, Wei N, Lv J, Chen W, Dai Z. Could preprocedural ultrasound increase the first-pass success rate of neuraxial anesthesia in obstetrics? A systematic review and meta-analysis of randomized controlled trials. J Anesth. 2020;34(3):434-44. [PubMed ID: 32133540]. https://doi.org/10.1007/s00540-020-02750-6.
-
40.
Rokhtabnak F, Sayad S, Izadi M, Djalali Motlagh S, Rahimzadeh P. Pain Control After Mastectomy in Transgender Patients: Ultrasound-guided Pectoral Nerve Block II Versus Conventional Intercostal Nerve Block: A Randomized Clinical Trial. Anesth Pain Med. 2021;11(5). e119440. [PubMed ID: 35070905]. [PubMed Central ID: PMC8771815]. https://doi.org/10.5812/aapm.119440.
-
41.
Elkhashab Y, Wang D. A Review of Techniques of Intercostal Nerve Blocks. Curr Pain Headache Rep. 2021;25(10):67. [PubMed ID: 34738179]. https://doi.org/10.1007/s11916-021-00975-y.
-
42.
Shirozu K, Kuramoto S, Kido S, Hayamizu K, Karashima Y, Hoka S. Hematoma After Transversus Abdominis Plane Block in a Patient With HELLP Syndrome: A Case Report. A A Case Rep. 2017;8(10):257-60. [PubMed ID: 28252541]. https://doi.org/10.1213/XAA.0000000000000487.
-
43.
Tran DQ, Bravo D, Leurcharusmee P, Neal JM. Transversus Abdominis Plane Block: A Narrative Review. Anesthesiology. 2019;131(5):1166-90. [PubMed ID: 31283738]. https://doi.org/10.1097/ALN.0000000000002842.
-
44.
Said AM, Balamoun HA. Continuous Transversus Abdominis Plane Blocks via Laparoscopically Placed Catheters for Bariatric Surgery. Obes Surg. 2017;27(10):2575-82. [PubMed ID: 28389846]. https://doi.org/10.1007/s11695-017-2667-9.
-
45.
Furuya T, Kato J, Yamamoto Y, Hirose N, Suzuki T. Comparison of dermatomal sensory block following ultrasound-guided transversus abdominis plane block by the lateral and posterior approaches: A randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2018;34(2):205-10. [PubMed ID: 30104830]. [PubMed Central ID: PMC6066880]. https://doi.org/10.4103/joacp.JOACP_295_15.
-
46.
Aditianingsih D, Anasy N, Tantri AR, Mochtar CA; Pryambodho. A randomized controlled trial on analgesic effect of repeated Quadratus Lumborum block versus continuous epidural analgesia following laparoscopic nephrectomy. BMC Anesthesiol. 2019;19(1):221. [PubMed ID: 31805855]. [PubMed Central ID: PMC6894195]. https://doi.org/10.1186/s12871-019-0891-7.
-
47.
Dam M, Hansen CK, Poulsen TD, Azawi NH, Wolmarans M, Chan V, et al. Transmuscular quadratus lumborum block for percutaneous nephrolithotomy reduces opioid consumption and speeds ambulation and discharge from hospital: a single centre randomised controlled trial. Br J Anaesth. 2019;123(2):e350-8. [PubMed ID: 31153628]. [PubMed Central ID: PMC6676058]. https://doi.org/10.1016/j.bja.2019.04.054.
-
48.
Kilic E, Bulut E. Quadratus Lumborum Block III for Postoperative Pain After Percutaneous Nephrolithotomy. Turk J Anaesthesiol Reanim. 2018;46(4):272-5. [PubMed ID: 30140533]. [PubMed Central ID: PMC6101721]. https://doi.org/10.5152/TJAR.2018.92331.
-
49.
Tanaka N, Kitazawa T, Mitani S, Suzuka T, Kadoya Y, Kawaguchi M. Anesthetic management using a combination of anterior quadratus lumborum block and erector spinae plane block for robot-assisted partial nephrectomy: two case reports. JA Clin Rep. 2020;6(1):65. [PubMed ID: 32815006]. [PubMed Central ID: PMC7438421]. https://doi.org/10.1186/s40981-020-00371-2.
-
50.
Aoyama Y, Sakura S, Abe S, Tadenuma S, Saito Y. Continuous quadratus lumborum block and femoral nerve block for total hip arthroplasty: a randomized study. J Anesth. 2020;34(3):413-20. [PubMed ID: 32232659]. https://doi.org/10.1007/s00540-020-02769-9.
-
51.
Tulgar S, Kose HC, Selvi O, Senturk O, Thomas DT, Ermis MN, et al. Comparison of Ultrasound-Guided Lumbar Erector Spinae Plane Block and Transmuscular Quadratus Lumborum Block for Postoperative Analgesia in Hip and Proximal Femur Surgery: A Prospective Randomized Feasibility Study. Anesth Essays Res. 2018;12(4):825-31. [PubMed ID: 30662115]. [PubMed Central ID: PMC6319070]. https://doi.org/10.4103/aer.AER_142_18.
-
52.
Deng W, Long X, Li M, Li C, Guo L, Xu G, et al. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain management after laparoscopic colorectal surgery: A randomized controlled trial. Medicine (Baltimore). 2019;98(52). e18448. [PubMed ID: 31876726]. [PubMed Central ID: PMC6946210]. https://doi.org/10.1097/MD.0000000000018448.
-
53.
Lai R, Luo Q, Lai J, Lu X, Xu M. Ultrasound-guided quadratus lumborum block for perioperative analgesia in robot-assisted partial nephrectomy: a randomized controlled trial. Trials. 2021;22(1):840. [PubMed ID: 34819150]. [PubMed Central ID: PMC8611864]. https://doi.org/10.1186/s13063-021-05815-3.
-
54.
Blanco R, Ansari T, Girgis E. Quadratus lumborum block for postoperative pain after caesarean section: A randomised controlled trial. Eur J Anaesthesiol. 2015;32(11):812-8. [PubMed ID: 26225500]. https://doi.org/10.1097/EJA.0000000000000299.
-
55.
Blanco R, Ansari T, Riad W, Shetty N. Quadratus Lumborum Block Versus Transversus Abdominis Plane Block for Postoperative Pain After Cesarean Delivery: A Randomized Controlled Trial. Reg Anesth Pain Med. 2016;41(6):757-62. [PubMed ID: 27755488]. https://doi.org/10.1097/AAP.0000000000000495.
-
56.
Balocco AL, Lopez AM, Kesteloot C, Horn JL, Brichant JF, Vandepitte C, et al. Quadratus lumborum block: an imaging study of three approaches. Reg Anesth Pain Med. 2021;46(1):35-40. [PubMed ID: 33159007]. https://doi.org/10.1136/rapm-2020-101554.
-
57.
Ueshima H, Otake H, Lin JA. Ultrasound-Guided Quadratus Lumborum Block: An Updated Review of Anatomy and Techniques. Biomed Res Int. 2017;2017:2752876. [PubMed ID: 28154824]. [PubMed Central ID: PMC5244003]. https://doi.org/10.1155/2017/2752876.
