1. Background
Pain, an unpleasant sensation that may vary in origin, intensity and duration, can be classified into the following types: nociceptive or transient, inflammatory, neuropathic, and functional (1).
Gabapentinoids are a new class of anticonvulsants that offer an exciting therapeutic approach to the treatment of different types of pain (2-4). Pregabalin, a novel anticonvulsant agent, has been evaluated in multiple clinical trials for pain treatment involving peripheral neuropathy, fibromyalgia, and irritable bowel syndrome (2, 3, 5, 6). Although a systemic review of clinical studies denies the effectiveness of pregabalin as analgesic in acute pain treatment (3), several studies have demonstrated its analgesic effect on acute, postoperative pain (7, 8) and in animal models of transient and visceral pain (9-14). Pregabalin is an interesting compound for visceral pain treatments such as irritable bowel syndrome (5). It decreases colonic nociceptive threshold (12) and modulates descending pathway involved in visceral pain transmission (15); the underlying mechanism(s) may be through the contribution of different neurotransmitters and receptors.
NMDA receptors (NMDARs) are known to be involved in pain associated with peripheral tissue or nerve injury (16-20). Continuous activation of NMDARs causes the spinal cord neurons to become more responsive to inputs, leading to the central sensitization component of chronic pain. The other component, which is defined as peripheral sensitization, consists of acquired increased excitability of sensory nerves or a decreased threshold of nociceptor activation (1, 4, 18, 20-22). NMDARs are found in both the enteric nervous system and the peripheral nervous system (23), so peripheral NMDARs become the target of NMDA antagonists, which may act as analgesics (19). In studies on visceral pain, the role of peripheral NMDARs was considered essential in acute nociceptive inputs from viscera (18). Overall, the role of NMDARs in peripheral sensitization and the location of these receptors on afferent somatic and visceral nerve axons suggest a potential approach to pain treatment (18).
2. Objectives
In our previous studies, we found a different antinociceptive potency of pregabalin in tail flick, hot plate, and visceral pain induced by acetic acid (11, 13, 14, 24). The hot plate test assesses acute pain by spinal and supra spinal mechanism and acetic acid induced visceral pain screens both the peripheral and central mechanisms (25). Because the involvement of NMDA receptors has not been determined yet, in this study we aimed at examining the probable involvement of NMDA receptors in pregabalin induced antinociception in acute thermal and visceral pain and determining the difference between hot plate, and writhing test in evaluation of this contribution.
3. Methods
3.1. Animals
Male Swiss albino mice (n = 160), weighing 25 to 35 g were housed 4 or 5 per cage at a controlled temperature (22 ± 2°C) on a 12-hour light-dark cycle. The animals had free access to food and water and were used for only 1 procedure before being humanely killed under anesthesia. The experiments were performed on the light cycle between 8 and 12 AM. The study protocol was approved in March 2011 by the research ethics committee of Kerman University of Medical Sciences (Ka-92/312) in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85 - 23, revised 1985).
3.2. Drugs
The drugs used included pregabalin (Hetero Drugs Limited, India), N-methyl-D-aspartic acid NMDA (Sigma-Aldrich,USA), and dizocilpine hydrogen maleate MK801 (Sigma-Aldrich, USA). All drugs were freshly dissolved in normal saline and were injected intraperitoneally (i.p.) 15 minutes before pregabalin or normal saline. The acetic acid (Sigma) was dissolved in distilled water.
3.3. Hot Plate Test
The hot plate test assesses the supra spinal response to a thermal noxious stimulus (25). The testing device assesses the antinociceptive effect of drugs by measuring the response latency in the animal. It consisted of an electrically heated surface and an open Plexiglass tube (30 cm high and 20 cm in diameter) to contain the animals. The heated surface was maintained at 55 + 0.5°C. Each animal was smoothly placed on the hotplate. The time before each mouse showed symptoms of feeling pain such as lifting or licking their hind or front paw or jumping out of the cylinder, which occurred very rarely, was measured in seconds and referred to as the hotplate latency time. The reaction time of each animal was tested at least twice before the experiment to exclude those with predrug latencies less than 2 and more than 5 seconds. To prevent injury to the animals, they were removed after a latency time of 15 seconds (cutoff = 15 seconds). The animals were randomly assigned to experimental groups of 6 mice. The latency times were measured once before and at the 30th and 60th minutes after drug injection to determine MPE30% and MPE60%.
3.4. Acetic Acid-Induced Writhing Test
The animals were placed in individual transparent boxes for 30 minutes to adjust to surroundings. Acetic acid 0.6% was prepared freshly. The animal was restrained and exposed ventrally, and an acetic acid at a dose of 10 mL/kg of body weight was injected intraperitoneally (i.p.) in lower right quadrant of abdomen, using insulin syringe at angle 30 grade, dept5 mm. Then, visceral pain was measured by counting the number of writhing reflexes for 30 minutes in experimental groups of 8 mice. The writhing reflexes were characterized by the presence of an abdominal contraction associated with inward outstretching of the hind limbs, a hind paw reflex, or a whole or back body extension. The investigator was blind to the drug injected.
3.5. Procedure
The study comprised of different groups as follow:
1) Control or untreated mice that received normal saline.
2) Pregabalin groups, which received doses of 100 mg/kg or 5 mg/kg, i.p. of pregabalin, according to the type of test (11, 13, 24).
3) MK801 groups that received doses of 0.02 and 0.05 mg/kg, i.p. (MK.02 andMK.05) (13).
4) NMDA groups that received doses of 15 and 30 mg/kg, i.p. (NMDA15 and NMDA30) (13).
5) (pg + MK) groups that received doses of 0.02 and 0.05 mg/kg, i.p. 15 minutes before the injection of pregabalin (pg + MK.02), (pg + MK.05).
6) (pg + NMDA) groups that received doses of 15 and 30 mg/kg, i.p. 15 minutes before the injection of pregabalin (pg + NMDA30), (pg + NMDA15).
In the writhing test, 15 minutes after administration of saline or drugs, acetic acid at 0.6% was administered. Next, the number of abdominal contractions was counted during the 30 minutes of the test administration. The antinociception was quantified as the percent effect in reduction in the number of writhes produced after each dose of the drug was injected: (E%) = [Number of writhes in control - Number of writhes in determined dose / Number of writhes in control] × 100.
In the hot plate assay, the latency times were measured immediately after drug or saline injection, and antinociception was quantified as the percentage of maximal possible effect at the 30th and 60th minutes after drug injection: (MPE30% and MPE60%) = [(T1 - T0)/ T2 - T0)] × 100. T1 was latency time at 30th and 60th minutes after drug administration. T0 and T2 were predrug latency time and cutoff time, respectively.
3.6. Statistical Analysis
Data were expressed as mean ± SEM of 6 mice in the hot plate assay or 8 mice in the writhing test. One-way analysis of variance (ANOVA), followed by Tukey’s test was used to evaluate the significant differences between E% and MPE% among the treated groups. Statistical analysis was conducted using SPSS software Version 15. P < 0.05 was considered statistically significant.
4. Results
4.1. The Effect of NMDARs Ligands Pretreatment on Antinociception of Pregabalin in Hot Plate Test
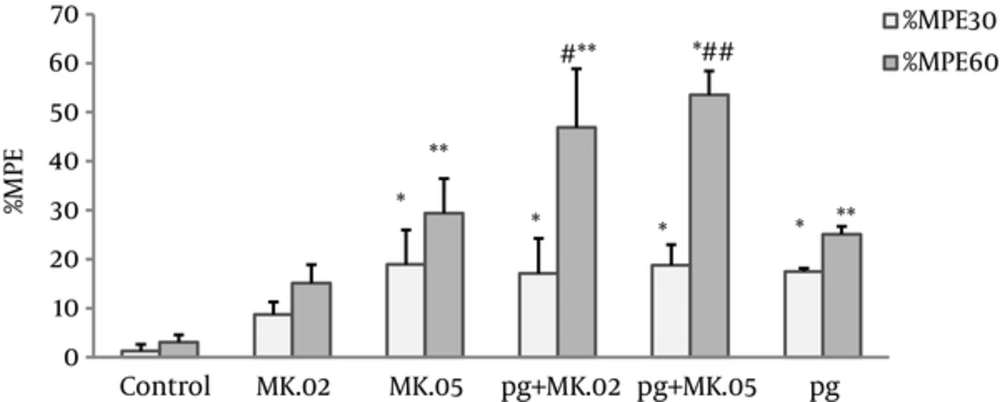
Our results revealed that both pregabalin (100 mg/kg) and MK801 (0.02 and 0.05 mg/kg), alone and in combination, significantly increased the antinociceptive effect at the 30th and 60th minutes after injection compared with the control group (Figure 1). Also, the MPE60% of coadministration of pregabalin with MK801 (0.02 and 0.05 mg/kg) was significantly higher than pregabalin alone (P < 0.05 and P < 0.005, respectively), but no differences were observed in the MPE30% of these MK801 pretreated group compared with pregabalin alone (Figure 1).
Maximum possible effect (MPE%) was measured at the 30th and 60th minutes of the pregabalin (100 mg/kg, i.p), MK801 (0.02 and 0.05 mg/kg, i.p), and combination groups. In the MK.02 + pg and MK.05 + pg groups, the MK801 was injected 15 minutes before the pregabalin. The data are expressed as the mean ± S.E.M of 6 mice. *P < 0.05 and **P < 0.001 compared to controls. #P < 0.05 and ##P < 0.005 compared to pregabalin.
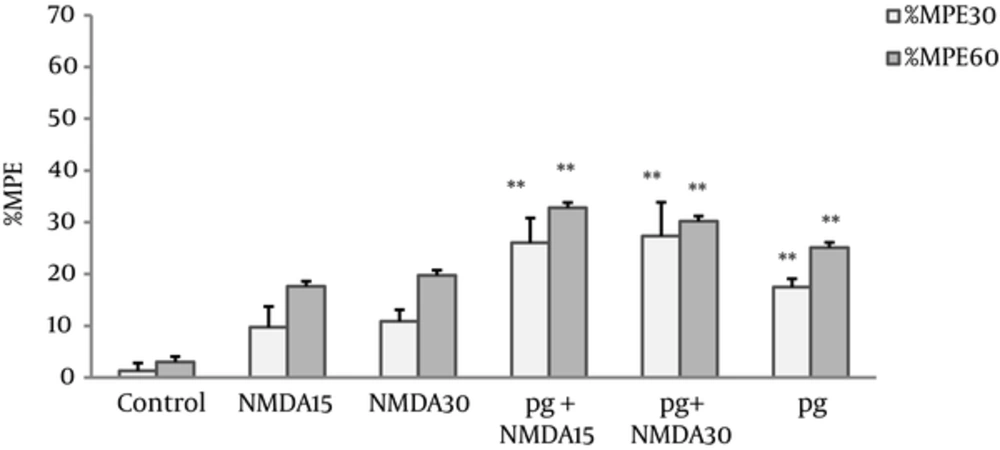
The MPEs% of NMDA group at doses of 15 and 30 mg/kg showed no significant differences compared with the control group. The MPEs% NMDA pretreated groups (pg + NMDA15 and pg + NMDA30) increased significantly (P < 0.005) compared with the controls, however, no difference was observed in comparison with pregabalin (Figure 2).
Maximum possible effect (MPE%) was measured at the 30th and 60th minutes of the pregabalin (100 mg/kg, i.p), NMDA (15 and 30 mg/kg, i.p.), and combination groups in the hot plate test. In the pg + NMDA15 and pg + NMDA30 groups, the NMDA was injected 15 minutes before the pregabalin. The data are expressed as the mean ± S.E.M of 6 mice. **P < 0.005 compared to control.
4.2. The Effect of Pretreatment with NMDARs Ligands on Antinociception of Pregabalin in the Acetic Acid Induced Writhing Assay
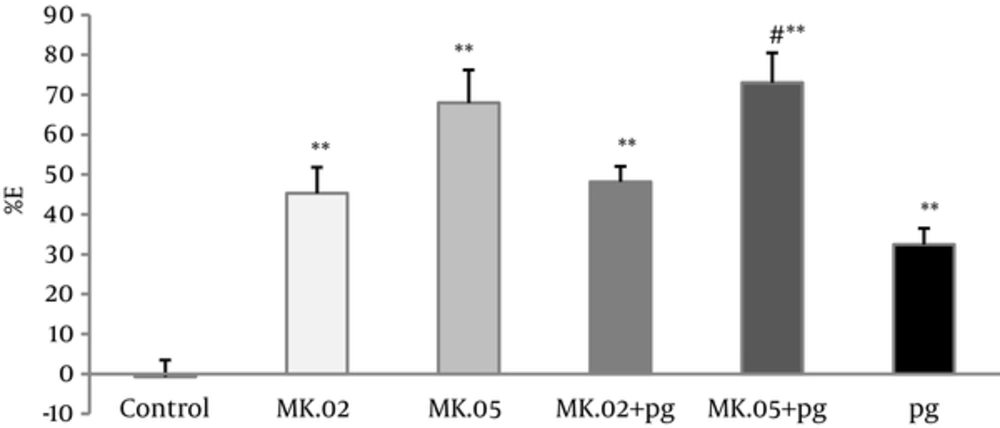
Pregabalin at 5 mg/kg and MK801 (0.02 and 0.05 mg/kg) reduced the number of abdominal contractions significantly in the acetic acid induced writhing assay compared with the controls (P < 0.005). Moreover, the antinociceptive effect of the pretreated groups with MK801 (pg + MK.02, pg + MK.05) showed a dose-dependent increase compared with the controls (P < 0.005).
The number of abdominal contractions of pretreatment with of MK801 (0.05 mg/kg) in pg + MK.05 group was reduced significantly compared with pregabalin group alone (P < 0.05), however, no difference was observed between pg+MK.02 and pregabalin alone (Figure 3).
Percent of effect (E%) was calculated in 30 minutes of writhing test of pregabalin (5 mg/kg, i.p), MK801 (0.02 and 0.05 mg/kg, i.p), and combination groups. In the MK.02 + pg and MK.05 + pg groups, the MK801 was injected 15 minutes before the pregabalin. The data are expressed as the mean ± S.E.M of 8 mice. **P < 0.005 compared to control. #P < 0.05 compared to pregabalin.
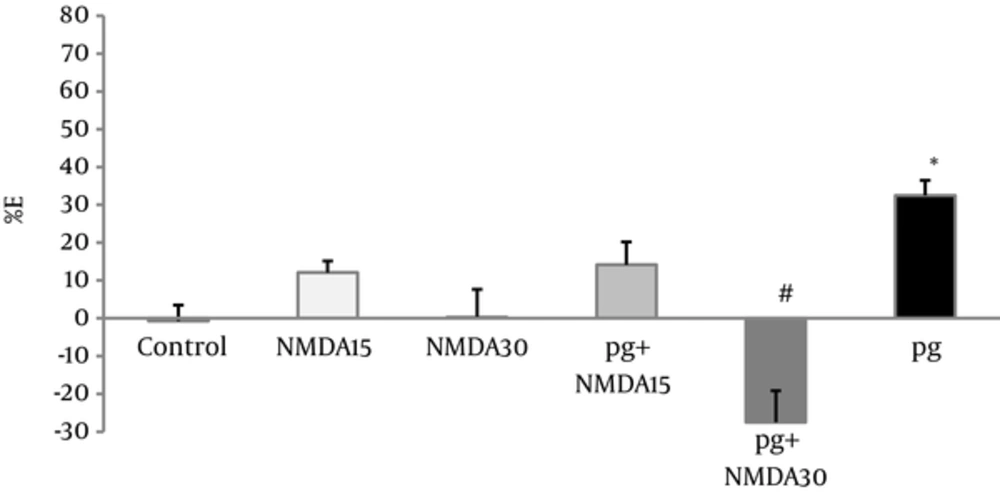
NMDA administration (15 and 30 mg/kg) did not produce any significant analgesic effects, but pretreatment of only high dose of NMDA (30 mg/kg) decreased the visceral pain antinociceptive effect of pregabalin (P < 0.001), (Figure 4).
Percent of effect (E%) was calculated in the 30th minute of writhing test of pregabalin (5 mg/kg, i.p), NMDA (15 and 30 mg/kg, i.p), and combination groups. In the pg+NMDA15 and pg + NMDA30 groups, the NMDA was injected 15 minutes before the pregabalin. The data are expressed as the mean ± S.E.M of eight mice. *p<0.05 compared to control, #p<0.001 compared to pregabalin.
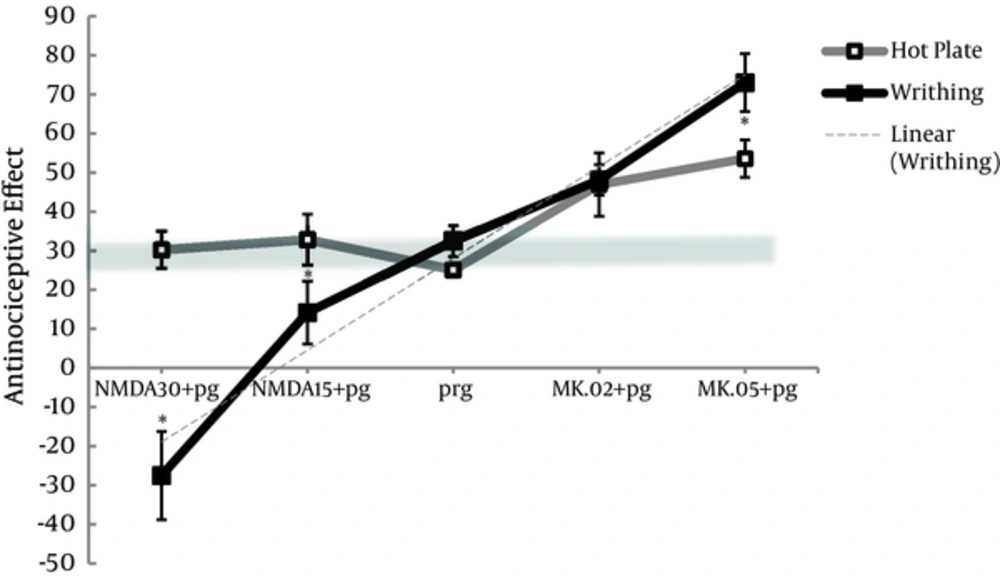
4.3. The Difference Between Hot Plate and Writhing Test in the Assessment of the Effect of NMDAR Ligands on Pregabalin Antinociception
Based on material and methods, the chosen doses of 100 and 5 mg/kg of pregabalin that produced almost 30% of antinociceptive effect in respective hot plate test and writhing test shaped the tick gray line of reference in Figure 5. Antinociceptive effect of pregabalin or NMDA alone was similar between hot plate and writhing test, and no distinct differences were observed in pretreated group of (MK.02 + pg) between the 2 tests (Figure 5). However, MK801 alone produced (P < 0.05) more antinociceptive effect in writhing test (E% = 45.2 ± 6.5 and E% = 68.0 ± 8.2, respectively) compared to hot plate (15.1 ± 1.5 and 29.3 ± 7.1, respectively) (Figures 1 and 3).
E% and MPE% were shown for pregabalin 5 or 100 mg/kg, i.p., respectively and combination groups with NMDA (15 and 30 mg/kg, i.p.) and MK801 (0.02 and 0.05 mg/kg, i.p.) Pretreatment with NMDA and MK801 in the pg+NMDA15, pg+NMDA30, and MK.05+pg groups produced remarkable different antinociception between hot plate and writhing test. The data are expressed as the mean ± S.E.M. *p<0.05 compared to hot plate test.
When pregabalin was pretreated with 0.05 mg/kg of MK801 (MK.05 + pg), the antinociceptive effect produced in writhing test was significantly increased compared to that produced in hot plate test (P < 0.05). Furthermore, NMDA at both doses (15 and 30 mg/kg) decreased (P < 0.05) the antinociceptive effect of pretreated groups (pg + NMDA15 and pg + NMDA30) in writhing test compared to hot plate (Figure 5).
In writhing test, the pretreatment of high dose of 30 mg/kg of NMDA decreased the antinociception of pregabalin from 30% to -30%, and pretreatment of high dose of 0.05 mg/kg of MK801 increased that effect from 30% to 70%, producing a linear trend model (r2 = 0.964).
5. Discussion
The results of the present study revealed the antinociceptive effect of pregabalin in hot plate and writhing test. MK801, a non-competitive NMDA receptor antagonist, indicated analgesic effect at a dose of 0.05 mg/kg (i.p.). MK801 pretreatment prior to pregabalin significantly increased the analgesic effect of pregabalin compared to controls and pregabalin alone.
We have previously reported the antinociceptive effect of pregabalin in tail flick, hot plate, and acetic acid induced visceral pain (11, 13, 14, 24). In addition, others reported the beneficial effects of pregabalin in inflammatory and neuropathic pain, considering its multimodal analgesic effect (9, 10, 26, 27). Although our results suggest that MK801 posses the analgesic activity in both acute and visceral pain, there are controversial reports on MK801 analgesic activity in acute pain. This controversy depends on dose and the type of pain assessment. In tail flick, same doses of MK801 and NMDA did not have any effect because tail flick test response is a spinal reflex to thermal nociceptive stimulus and probably could measure antinociceptive effect of higher doses (13). Our results are in agreement with those of Nakama-Kitamura and Al-Amin et al. who showed a dose dependent antinociceptive effect of MK801 in hot plate test in mice and rats, respectively (28, 29). Moreover, other investigators reported that MK801 decreased abdominal contractions in acetic acid induced writhing in mice (30) and it abolished the visceral pain induced by noxious and non-noxious stimuli of colorectal distention in rats, indicating that MK801 analgesic activity is mediated through both central and peripheral mechanisms (31, 32). The mediation of NMDARs antagonists on human visceral pain was demonstrated when Ketamine was shown to be more effective in relieving visceral pain than somatic pain (17). Injection of glutamate or NMDA into the rat hind paw produced mechanical hyperalgesia and increased pain behavior to radiant heat (33, 34).
Pretreatment with MK801 significantly increased the antinociception activity of pregabalin in both acute and acetic acid induced visceral pain, indicating the involvement of NMDA receptors in both acute and visceral pain processes. Also, in our previous study, MK801 (0.05mg/kg/i.p.) increased antinociceptive effect of pregabalin in tail flick model of acute pain (13). In agreement to our results, others have reported that systemic administration of NMDAR antagonists including MK801 potentiates the analgesic effect of low doses of morphine in tail flick test (35), and prolongs the morphine analgesic effect in hot plate test (36). Likewise, subanalgesic doses of Ketamine enhanced the antinociceptive effect of morphine in the writhing test (30).
To the best of our knowledge, no study has yet assessed the effect of MK801 (or any other NMDAR antagonist) on pregabalin antinociception in either hot plate or acetic acid induced visceral pain. However, MK801 pretreatment (0.05 mg/kg) produced a synergistic effect on gabapentin antinociception in formalin test in rats without effecting motor coordination (37-39).
The activation of peripheral and central NMDARs is related to different types of pain production and pathways (18). Moreover, NMDA did not change the thermal pain threshold, while injection of glutamate or NMDA into the rat hind paw produced mechanical hyperalgesia and increased pain behavior to radiant heat (33, 34). In another study on NMDAR-knockdown mice, the behavioral responses to thermal nociception (tail flick and hot plate) were unchanged, as opposed to the behavioral response to inflammatory pain, for which significant antinociception was detected (16). Thus, in agreement with our results, these investigations demonstrated the involvement of NMDR in inflammatory, but not transient pain stimulus (18, 40). Accordingly, in our study, MK801 suggested a modest effect of 30% in the hot plate supraspinal integrated response, and a 50% to 70% antinociceptive effect in the writhing test, which represented a central complex response measurement. For that reason, NMDA reduced the antinociceptive effect of pregabalin in writhing test, but not in hot plate test. In the same way, after pretreatment with 30 mg/kg of NMDA, the number of abdominal contractions was reduced in such a way to make negative values of E%, generating a hyperalgesic effect. The development and maintenance of visceral hypersensitivity was previously shown to be NMDA receptor mediated (17). Also, in an inflammatory model of cyclophosphamide-induced visceral cystitis pain, the expression of NMDARs was increased in hypersensitive rats (41).
Involvement of NMDA ligands in antinociception of pregabalin was well- recognized using acetic acid induced abdominal contractions because NMDA pretreatment decreased the analgesic effect of pregabalin in acetic acid induced visceral pain, and MK801 pretreatment increased pregabalin antinociception. Thus, these data suggest that pregabalin antinociception in visceral pain is mediated partly through the involvement of NMDAR on the terminals of primary efferent nerves innervating visceral and colon (2, 4). Pregabalin suppresses spinal hyperactivity via the inhibition of both pre- and post-synaptic NMDARs (42). At the cerebral level of pain perception, pregabalin inhibits stimulus-evoked glutamate release (43). In cell culture studies, pregabalin action depends on NMDA receptor activation and was proposed to act as an indirect antagonist, reducing the levels of intracellular d-serin, a known co-agonist of NMDARs (44, 45).
Our study proposed that inhibition of NMDAR by MK801 results in marked increase in pregabalin antinociception in writhing test (E% = 70), but not in hot plate test. This outcome suggests the involvement of NMDAR in visceral pain nociception through the presence of NMDARs on primary afferent nerve terminals, innervating viscera and the colon (40). Moreover, we have previously reported that ED50 in writhing test is almost 15 times lower than that of hot plate (9, 24). Although pregabalin antinociception in visceral pain could be mediated through NMDA receptors, other mechanism(s) may be involved in pregabalin visceral antinociception including glutaminergic mechanisms (5, 15, 44).
In conclusion, our results proposed the potent antinociceptive effect of pregabalin in acetic acid induced visceral pain and low antinociceptive property in hotplate test. In addition, our results suggested that MK801 significantly increased the pregabalin antinociception in visceral pain through the inhibition of NMDA receptor, indicating that pregabalin antinociception in acetic acid induced visceral pain is at least partly mediated through the involvement of NMDA receptors. Comparison of the writhing and hot plate tests suggested that the writhing test was more appropriate to assess the involvement of NMDARs.