Abstract
Background:
Postoperative pain is one of the most common problems after hernia repair. Decrease in postoperative pain accelerates functional recovery, decreases duration of hospital stay and postoperative morbidity.Objectives:
To compare postoperative analgesic effect of infiltration of magnesium versus bupivacaine into incision of inguinal hernia repair.Patients and Methods:
In a double blind clinical trial, 80 patients’ candidates for elective inguinal hernia repair were enrolled. Right before closure of incision, in Bupivacaine group 5 mL Bupivacaine 0.5% added to 5 mL normal saline and in Magnesium group, 10 mL Magnesium sulfate 20% was infused subcutaneously. Pain score was measured using numeric rating score (NRS) at 1, 3, 6, 12 and 24 hours postoperatively. If NRS was above 3, 1 mg morphine was administered as rescue analgesic until patient felt comfortable or NRS < 3.Results:
Postoperative pain scores at 1 and 3 hours were not significantly different between bupivacaine and magnesium groups (P = 0.21, 0.224; respectively). However, at 6 (P = 0.003), 12 (P = 0.028) and 24 (P = 0.022) hours postoperative, pain score (NRS) was significantly lower in bupivacaine group. Number of patients needed at least 1 dose of rescue morphine (P = 0.001), mean number of episodes asked for morphine during next 24 hours (P = 0.001) and total dose of morphine requirement (P = 0.01) were significantly lower in bupivacaine group.Conclusions:
Magnesium infiltration did not decrease total dose and number of episodes needed for morphine rescue analgesic. Bupivacaine infiltration into surgical site was more effective than magnesium sulfate infiltration in postoperative pain control.Keywords
1. Background
About 10% of people are involved with hernia during their life-time. Abdominal hernia is a common disease, which occurs in 1.7% of all ages and in 4% of age over 45 (1). Inguinal hernia is responsible for 75% of hernia. Risk of development of inguinal hernia is 24 - 27% in males and 3% in females (2).
Postoperative pain is one of the most common problems after hernia repair, and up to 80% of patients require opioid analgesia after laparoscopy (3). Decrease in postoperative pain accelerates functional recovery, decreases duration of hospital stay and postoperative morbidity. Uncontrolled postoperative pain induced chronic pain is a common complication after inguinal hernia repair (4). Although opioids are the mainstay of postoperative pain control, high dose of opioids has several adverse effects such as respiratory depression, ileus, nausea and vomiting. On the other hand, decrease in opioid dose would increase the rate of postoperative pain in patients. Non-steroidal anti-inflammatory drugs (NSAIDs) are another replacement to opioids; however, NSAIDs are associated with many adverse effects, including decreased hemostasis, renal dysfunction and gastrointestinal hemorrhage.
Various modalities of treatment have been proposed and found to be variably effective for inguinal hernia postoperative pain control. Ilioinguinal and iliohypogastric nerves block (5) and intraperitoneal bupivacaine injection are various modalities to control pain of hernia repair. Local infiltration, subfascial or subcutaneous administration of tramadol, bupivacaine, lidocaine, or meperidine in to incision site was also attempted in some studies. Wound infiltration with local anesthetics is often used for pain relief after inguinal hernia surgery. Topical infiltrative bupivacaine is even more effective than intravenous meperidine for postoperative analgesia after inguinal herniorrhaphy (6). However, controversy still exists and local anesthetic infiltration has not been proved as an ultimate method (7).
Magnesium sulfate is adjuvant for pain control, which antagonizes calcium similar to NMDA antagonists (8, 9). Magnesium and Bupivacaine both offered safe and low cost drugs to decrease postoperative pain and analgesic consumption and have been used as effective adjuvants in postoperative pain management (10). However, Magnesium sulfate infiltration has not been used for postoperative inguinal hernia pain control.
2. Objectives
The aim of this study was to compare analgesic effect of magnesium versus Bupivacaine infiltration into incision of inguinal hernia repair to control postoperative pain.
3. Patients and Methods
This study was reviewed and approved by Shahid Beheshti University of Medical Sciences Review Board and Ethics Committee. Information about trial was given comprehensively both orally and in written form. All patients gave their informed written consents prior to their inclusion in the study.
3.1. Patient Selection
In a double blind clinical trial, 80 patients candidates for elective inguinal hernia repair enrolled in this study and randomly assigned to one of magnesium or Bupivacaine group based on accidental numbers assigned by computer to each case. Inclusion criteria were unilateral inguinal hernia, ASA classes I and II and age between 18 - 70 years. Exclusion criteria were severe cardiac, respiratory and renal disease, pregnancy, opioid tolerant patients and neuromuscular diseases. Study was performed between 2013 and 2014.
Study was double blinded as physicians performing infiltration were blinded to the groups of patients as the anesthesiologist evaluating patients. Patients were also blinded to the drug administered. Drugs were delivered in the same size syringe and same color by the anesthesiologist performing spinal anesthesia (concealment allocation).
3.2. Anesthesia
Anesthesia was induced by the same method in all patients. Patients were monitored for electrocardiogram (ECG), heart rate, oxygen saturation (SPO2%), non-invasive blood pressure (NIBP) and end-tidal CO2 (ETCO2) after the entrance to the operation room. Patients were pre-oxygenated with 5 lit/min 100% O2 for 3 to 5 minutes. For premedication, fentanyl 2 µg/kg and midazolam 0.02 mg/kg administered. Anesthesia was induced by sodium thiopenthal 5 mg/kg, Lidocaine 1.5 mg/kg and cisatracurium 0.2 mg/kg. Intubation was performed under smooth direct laryngoscopy after 60 - 90 seconds. Endotracheal tube size was selected after laryngoscopy under direct visualization. Anesthesia was maintained using Isofluorane 1%, O2 50%/N2O 50%. No other opioid or analgesics was administered during anesthesia.
3.3. Magnesium and Bupivacaine Infiltration
All surgeries were performed using Lichtenstein method under general anesthesia. Just before closure of incision, in Bupivacaine group, 5 mL of Bupivacaine 0.5% added to 5 mL normal saline infiltrated subcutaneously. In Magnesium group, 10 mL Magnesium sulfate 20% infiltrated subcutaneously.
3.4. Postoperative Pain Measurement
A trained anesthesiologist blind to the groups of study assessed pain and analgesic consumption. Pain score was measured using numeric rating score (NRS) at 1, 3, 6, 12 and 24 hours postoperative. If NRS was above 3, 1 mg morphine was administered as rescue analgesic until patient felt comfortable or NRS < 3. All adverse effects including nausea vomiting and dizziness were recorded during 24 hours postoperatively. Other variables including number of patients needed at least 1 dose of rescue morphine, number of rescue analgesic request during next 24 hours and total dose of morphine requirement were measured and recorded in specified data sheet during next 24 hours.
Postoperative monitoring included noninvasive blood pressure, heart rate and pulse oximetry. Nausea was treated with metoclopramide (10 mg) and postoperative shivering was treated with a forced air warming blanket.
3.5. Statistical Analysis
Statistical analysis was performed using SPSS 20 (Chicago, IL, USA). The parametric variables were presented as mean ± SD and analyzed by student t-test; non-parametric variables were analyzed by Chi-Square or Mann-Whitney U-test. P < 0.05 was considered as statistically significant. Sample size was estimated using sample size calculator software with 95% confidence interval, P = 0.05 and power of 80% and difference between the two groups of 30% in primary outcome based on pilot study.
4. Results
In a randomized clinical trial, 80 patients were randomly assigned to one of the two groups of study. There was no significant difference between the two groups in demographic variables including age, sex and BMI (P > 0.05). Duration of operation was not significantly different between the two groups of study (P = 0.414) (Table 1).
Demographic Characteristics of Patients in the Two Groups of Study
| Bupivacaine (n = 40) | Magnesium | P Value | |
|---|---|---|---|
| N = 40 | |||
| Age, years | 37.2 ± 18.6 | 35.8 ± 16.5 | .295 |
| Gender, Male/Female | 35/5 | 33/7 | .531 |
| BMI, kg/m2 | 27.6 ± 2.4 | 28.2 ± 1.9 | .429 |
| Duration of Operation, min | 107 ± 22 | 104 ± 18 | .414 |
Postoperative pain score at different time points were compared between the two groups of study. Postoperative pain scores at 1 and 3 hours were not significantly different between bupivacaine and magnesium groups (P = 0.21, 0.224; respectively). However, at 6 (P = 0.003), 12 (P = 0.028) and 24 (P = 0.022) hours postoperative, pain score (NRS) was significantly lower in bupivacaine group (Table 2).
Comparison of Postoperative Pain Score at Different Time Points in the Two Groups
| Postoperative Time, hours | Bupivacaine (n = 40) | Magnesium (n = 40) | P Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| 1 | 1.6 ± 0.8 | (3 - 0) | 1.8 ± 0.7 | (3 - 0) | .21 |
| 3 | 2.4 ± 1.2 | (6 - 1) | 2.7 ± 1 | (5 - 1) | .224 |
| 6 | 3.1 ± 1 | (6 - 1) | 3.9 ± 1.3 | (7 - 2) | .003 |
| 12 | 2.7 ± 0.9 | (5 - 1) | 3.3 ± 1.2 | (6 - 1) | .028 |
| 24 | 2.5 ± 1.1 | (5 - 1) | 3.1 ± 1.1 | (6 - 1) | .022 |
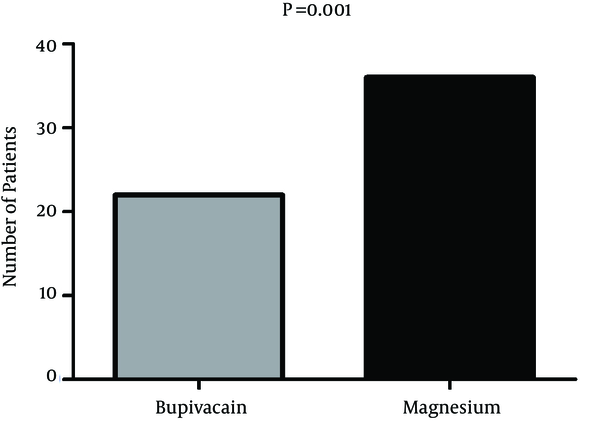
Comparison of number of patients needed at least 1 dose of rescue morphine was performed between the two groups. In magnesium group, 36 patients needed at least 1 dose of morphine and in Bupivacaine group 22 patients needed rescue analgesic, which was significantly lower in bupivacaine group (P = 0.001, chi-square test) (Figure 1).
Comparison of Number of Patients Needed at Least One Dose of Rescue Morphine
Mean ± SD number of episodes asked for morphine during next 24 hours were 0.9 ± 0.9 in Bupivacaine group and 1.6 ± 1 in magnesium group, which was significantly different (P = 0.001). Total dose of morphine requirement was 36 ± 12 mg in bupivacaine group and 64 ± 14 mg in Magnesium group, which was significantly lower in bupivacaine group (P = 0.01) (Table 3).
Comparison of Morphine Rescue Analgesic Consumption During 24 Hours Postoperation in the Two Groupsa
| Variables | Bupivacaine | Magnesium | P Value |
|---|---|---|---|
| Number of episodes asked for morphine, n | 0.9 ± 0.9 | 1.6 ± 1 | 0.001 |
| Total dose of morphine requirement, mg | 36 ± 12 | 64 ± 14 | 0.01 |
Postoperative nausea and vomiting were also compared between the two groups and 9 (22.5%) patients in bupivacaine and 15 patients (37.5%) in magnesium group had PONV, which was not significantly different (P = 0.143, chi-square test).
5. Discussion
In this study we compared injection of magnesium and bupivacaine into inguinal hernia surgical site before closure to control postoperative pain. Our results depicted superior effect of bupivacaine compared to magnesium in decreasing pain and morphine rescue analgesic during next 24 hours after the operation.
Inguinal hernia is accompanied with moderate to severe postoperative pain. Postoperative pain increased in both groups at 1 and 3 hours postoperation, which was not significantly different in the both groups. At these two time points, pain reached to maximum in the both groups, which is probably due to release of inflammatory cytokines at surgical site. However, at 6, 12 and 24 hours postoperation, pain score decreased in the both groups, which is probably due to decrease in inflammatory response and blocking nociceptive pain transmission. Interestingly, at these latter time points, pain score was significantly lower in bupivacaine compared to magnesium group.
The mainstay of current postoperative pain management after inguinal hernia is local infiltration of local anesthetics and postoperation rescue analgesics, mainly opioids. Studies showed that wound infiltration with long-acting local anesthetics resulted in low pain scores after hernia surgery (11, 12). Bupivacaine injection at the surgical site is proved to prolong the first time to analgesia, reduce early postoperative opioid requirements and lower pain in males undergoing open hernia repair (13, 14). Postoperative infiltration of the wound with 0.5% bupivacaine achieved superior analgesia compared with oral analgesics alone (15). However, none has ever compared local anesthetics with magnesium and therefore our study was unique in this regard.
Our results showed that magnesium is less effective than bupivacaine in controlling postoperative pain. Previous reports showed that magnesium is more effective in pain control when used as systemic adjuvants (16). This probably underlines the fact that Magnesium analgesic effect is more prominent in systemic injection than local infiltration. It seems that it has a central effect more than local anti-nociceptive effect. The analgesic effect of magnesium is mediated through NMDA receptors block at spinal cord level. Magnesium, a NMDA receptor antagonist, has been demonstrated to increase the analgesic effects of opioids, probably by limiting NMDA-mediated facilitating processes (17). Decrease in VAS score postoperatively is related to magnesium effect on pain pathway. Pastore et al. hypothesized that magnesium used as an adjunct to analgesia is based on a non-competitive antagonism towards the NMDA receptor and on the blocking of calcium channels, which prevents the mechanisms of central sensitization due to nociceptive stimulation of peripheral nerves (18).
Although magnesium effect as a sole local anesthetic is still debated, it is able to block morphine and its derivates tolerance through NMDA antagonism. Magnesium deficiency induces a sensitization of nociceptive pathways in the spinal cord, which involves NMDA and non-NMDA receptors (19). Albrecht et al. showed that perioperative intravenous magnesium reduces opioid consumption, and to a lesser extent, pain scores, in the first 24 hours postoperatively (20). However, our results showed that morphine consumption and number of episodes asking morphine analgesic were significantly higher in magnesium compared to bupivacaine group. This might be due to higher percentage of PONV in magnesium group compared to bupivacaine group. In future studies, preoperative and postoperative magnesium level in serum should be measured to prove these results in a clinical setting.
In conclusion, infiltration of bupivacaine into surgical site is more effective than magnesium sulfate in postoperative pain control. Magnesium infiltration did not decrease total dose and number of episodes asked for morphine rescue analgesic.
References
-
1.
de Goede B, Timmermans L, van Kempen BJ, van Rooij FJ, Kazemier G, Lange JF, et al. Risk factors for inguinal hernia in middle-aged and elderly men: results from the Rotterdam Study. Surgery. 2015;157(3):540-6. [PubMed ID: 25596770]. https://doi.org/10.1016/j.surg.2014.09.029.
-
2.
Primatesta P, Goldacre MJ. Inguinal hernia repair: incidence of elective and emergency surgery, readmission and mortality. Int J Epidemiol. 1996;25(4):835-9. [PubMed ID: 8921464].
-
3.
Goldstein A, Grimault P, Henique A, Keller M, Fortin A, Darai E. Preventing postoperative pain by local anesthetic instillation after laparoscopic gynecologic surgery: a placebo-controlled comparison of bupivacaine and ropivacaine. Anesth Analg. 2000;91(2):403-7. [PubMed ID: 10910857].
-
4.
Kurmann A, Fischer H, Dell-Kuster S, Rosenthal R, Audige L, Schupfer G, et al. Effect of intraoperative infiltration with local anesthesia on the development of chronic pain after inguinal hernia repair: a randomized, triple-blinded, placebo-controlled trial. Surgery. 2015;157(1):144-54. [PubMed ID: 25482469]. https://doi.org/10.1016/j.surg.2014.07.008.
-
5.
Baerentzen F, Maschmann C, Jensen K, Belhage B, Hensler M, Borglum J. Ultrasound-guided nerve block for inguinal hernia repair: a randomized, controlled, double-blind study. Reg Anesth Pain Med. 2012;37(5):502-7. [PubMed ID: 22705951]. https://doi.org/10.1097/AAP.0b013e31825a3c8a.
-
6.
Waechter FL, Sampaio JA, Pinto RD, Alvares-Da-Silva MR, Pereira-Lima L. A comparison between topical and infiltrative bupivacaine and intravenous meperidine for postoperative analgesia after inguinal herniorrhaphy. Am Surg. 2001;67(5):447-50. [PubMed ID: 11379646].
-
7.
Tong YS, Wu CC, Bai CH, Lee HC, Liang HH, Kuo LJ, et al. Effect of extraperitoneal bupivacaine analgesia in laparoscopic inguinal hernia repair: a meta-analysis of randomized controlled trials. Hernia. 2014;18(2):177-83. [PubMed ID: 23644775]. https://doi.org/10.1007/s10029-013-1100-0.
-
8.
Koinig H, Wallner T, Marhofer P, Andel H, Horauf K, Mayer N. Magnesium sulfate reduces intra- and postoperative analgesic requirements. Anesth Analg. 1998;87(1):206-10. [PubMed ID: 9661575].
-
9.
Kara H, Sahin N, Ulusan V, Aydogdu T. Magnesium infusion reduces perioperative pain. Eur J Anaesthesiol. 2002;19(1):52-6. [PubMed ID: 11913804].
-
10.
Bhatia A, Kashyap L, Pawar DK, Trikha A. Effect of intraoperative magnesium infusion on perioperative analgesia in open cholecystectomy. J Clin Anesth. 2004;16(4):262-5. [PubMed ID: 15261316]. https://doi.org/10.1016/j.jclinane.2003.08.012.
-
11.
Pettersson N, Berggren P, Larsson M, Westman B, Hahn RG. Pain relief by wound infiltration with bupivacaine or high-dose ropivacaine after inguinal hernia repair. Reg Anesth Pain Med. 1999;24(6):569-75. [PubMed ID: 10588564].
-
12.
Vintar N, Pozlep G, Rawal N, Godec M, Rakovec S. Incisional self-administration of bupivacaine or ropivacaine provides effective analgesia after inguinal hernia repair. Can J Anaesth. 2002;49(5):481-6. [PubMed ID: 11983663]. https://doi.org/10.1007/BF03017925.
-
13.
Hadj A, Hadj A, Hadj A, Rosenfeldt F, Nicholson D, Moodie J, et al. Safety and efficacy of extended-release bupivacaine local anaesthetic in open hernia repair: a randomized controlled trial. ANZ J Surg. 2012;82(4):251-7. [PubMed ID: 22510183]. https://doi.org/10.1111/j.1445-2197.2011.05754.x.
-
14.
El-Radaideh KM, Al-Ghazo MA, Bani-Hani KE. Combined subfascial and subcutaneous bupivacaine instillation for inguinal hernia wounds. Asian J Surg. 2006;29(4):242-6. [PubMed ID: 17098656]. https://doi.org/10.1016/S1015-9584(09)60096-8.
-
15.
Lau H, Patil NG, Lee F. Randomized clinical trial of postoperative subfascial infusion with bupivacaine following ambulatory open mesh repair of inguinal hernia. Dig Surg. 2003;20(4):285-9. [PubMed ID: 12748432]. https://doi.org/10.1159/000071187.
-
16.
Telci L, Esen F, Akcora D, Erden T, Canbolat AT, Akpir K. Evaluation of effects of magnesium sulphate in reducing intraoperative anaesthetic requirements. Br J Anaesth. 2002;89(4):594-8. [PubMed ID: 12393361].
-
17.
Richebe P, Rivat C, Laulin JP, Maurette P, Simonnet G. Ketamine improves the management of exaggerated postoperative pain observed in perioperative fentanyl-treated rats. Anesthesiology. 2005;102(2):421-8. [PubMed ID: 15681961].
-
18.
Pastore A, Lanna M, Lombardo N, Policastro C, Iacovazzo C. Intravenous infusion of magnesium sulphate during subarachnoid anaesthesia in hip surgery and its effect on postoperative analgesia: our experience. Transl Med UniSa. 2013;5:18-21. [PubMed ID: 23905078].
-
19.
Begon S, Pickering G, Eschalier A, Mazur A, Rayssiguier Y, Dubray C. Role of spinal NMDA receptors, protein kinase C and nitric oxide synthase in the hyperalgesia induced by magnesium deficiency in rats. Br J Pharmacol. 2001;134(6):1227-36. [PubMed ID: 11704642]. https://doi.org/10.1038/sj.bjp.0704354.
-
20.
Albrecht E, Kirkham KR, Liu SS, Brull R. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anaesthesia. 2013;68(1):79-90. [PubMed ID: 23121612]. https://doi.org/10.1111/j.1365-2044.2012.07335.x.