Abstract
Background:
Postoperative shivering is a major problem in children undergoing general anesthesia.Objectives:
The aim of the present study was to investigate the role of low-dose intravenous ketamine for prevention of shivering after induction of general anesthesia in children who had undergone tonsillectomy.Patients and Methods:
This was a randomized, double-blinded, placebo-controlled trial including 80 children, of American society of anesthesiologists (ASA) physical status I or II, scheduled for tonsillectomy under general anesthesia who were randomly assigned to an intravenous ketamine (0.5 mg/kg, n = 40; group K) group or matched dose placebo (n = 40; group N) group. Surgical and demographic data, unexpected side effects, and the occurrence of shivering for each child were assessed by a blinded observer at the following time points: T0, in the recovery room; T10, at 10 minutes; T20, at 20 minutes; T30, and at 30 minutes.Results:
With regards to the demographic and surgical data, no significant differences between the two study groups were observed (P ≥ 0.05). Shivering intensity in children who had received ketamine was significantly lower than children who had not received ketamine, at T0, T10, T20, and T30 after arrival (P < 0.05). There were no significant differences in hallucination, nausea, vomiting, hemodynamic dysfunction, blurred vision, and seizure in the K group compared with the N group (P ≥ 0.05).Conclusions:
Administration of intravenous ketamine at a dosage of 0.5 mg/kg immediately after anesthesia induction had a preventive effect on shivering intensity without hemodynamic alterations in children undergoing general anesthesia for tonsillectomy.Keywords
1. Background
Amongst major duties of physicians is to manage patients’ complaints. The main reason for morbidity in patients undergoing surgical procedures is post-operative complications (1). One of the most unpleasant complications occurring during the early phase of recovery following general anesthesia is shivering, which is known as clonic movements recovering five to thirty minutes after anesthesia cessation (2). Shivering is random spontaneous and asynchronous skeletal muscle contractions that increases the basal metabolism and is characterized to be a defense mechanism for regulation of temperature in adults (3). The incidence of shivering varies from 5% to 65% with general anesthesia, while it occurs in 57% and 30% of cases following regional and epidural anesthesia, respectively (4). Shivering causes physiological stress, which results in increased carbon dioxide production, oxygen consumption, cardiac output, intracranial and intraocular pressure, and other complications related to sympathetic stimulation (5). In this regard, an important aspect of patient care is the treatment and prevention of post-operative shivering.
Although various pharmacological agents have been used to prevent or treat the post-operative shivering, including alfentanil, sufentanil, ketanserin, physostigmine, nefopam, urapidil, doxapram, tramadol, nalbuphine, and pethidine, the ideal drug for this query has not been found (2-7). Among these drugs, pethidine has been shown to be one of the effective drugs. Nevertheless, some disadvantages including nausea, vomiting, hallucination and respiratory distress have been reported following the use of pethidine (2).
At a number of levels, N-methyl-d-aspartate (NMDA) receptor antagonists are likely to modulate thermoregulation. N-methyl-d-aspartate receptors, located at the dorsal horn of the spinal cord, play an important role in modulating transmission of nociceptive stimuli. In contrast to regional or local anesthesia, general anesthesia does not inhibit the peripheral ascending nociceptive transmission from the site of operation to the dorsal horn of the spinal cord (8). In this regard, NMDA receptors blockade may prevent central sensitization. Consequently, NMDA antagonists at sub-anesthetic doses block or inhibit central hypersensitivity (9). Ketamine, NMDA receptor competitive antagonist, has different characteristics such as cerebral vasodilatation, induction of relaxation of bronchial smooth muscle, amnesia, ability to increase intra-cranial pressure, cause transient, and marked increase of blood pressure by sympathetic system stimulation, and analgesia (10). Ketamine can likely control shivering, as a prophylactic agent (11).
Different doses of intravenous ketamine have been used to treat and prevent shivering during general anesthesia (11-14); however, the detailed influence of low dose intravenous ketamine to treat and prevent shivering in children undergoing general anesthesia has not been extensively examined. Side effects of ketamine have been reported mostly in high doses. Nevertheless, there is a consensus concern regarding the optimal dose for children.
2. Objectives
We administered an intravenous infusion of low dose intravenous ketamine for general anesthesia in children who were undergoing tonsillectomy. Thereafter, we aimed to evaluate the effects of this type of treatment on the prevention of shivering in children during general anesthesia.
3. Patients and Methods
The current study was a randomized, double-blinded, placebo-controlled trial performed at the anesthetic clinic of Peymanie hospital, which is a tertiary healthcare center affiliated with Jahrom University of Medical Sciences during November 2013 to December 2014. Eighty children (American society of anesthesiologist (ASA) physical status I or II), who were scheduled for tonsillectomy under general anesthesia, ranging in age from six to twelve years, were enrolled in this study, as shown in Figure 1. The study protocol was based on the tenets of the declaration of Helsinki and was approved by the institutional review board of the Jahrom University of Medical Sciences. We obtained the approval of the ethics committee of Peymanie hospital before the study, and all of the participants’ parents or guardians gave their written informed consent. Recurrent tonsillar infection was the indication for surgery. None of the participants had a history of bleeding disorders, known hypersensitivity to the study medication (ketamine), and signs of acute pharyngeal infection. Furthermore, none of the children underwent additional surgical procedures such as adenoidectomy or insertion of ventilation tubes.
Participation Flow Chart
The patients had been referred to the anesthetic center for pre-operative evaluation, one day before the operation. They were registered to participate in the study after referral by a physician who was not part of the study. Each participant selected a sealed envelope containing a number of admissions. The envelope was opened by a nurse who was blinded to the study groups. Randomization was done by a computer-based random digit generator. According to the random number tabulation, the sealed black and white boxes containing ketamine and placebo, respectively, were assigned to each child. Thus, the patients were randomly allocated to receive either ketamine (Ketalar, Parke-Davis, India) at a dosage of 0.5 mg/kg (40 patients; group K) or matched placebo (normal saline) (40 patients; group N).
Six and three hours before induction of anesthesia, all children had fasted for solids and clear fluids, respectively. Although no pre-medications were given, the children received Emila topical cream an hour before induction of anesthesia to facilitate venous cannulation. A surgeon who was blinded to the study protocol operated on all the patients and the same and standardized general anesthetic protocol was used for all patients. Children were monitored by an electrocardiogram, for heart rate (HR), blood pressure (BP), and oxygen saturation before the induction of general anesthesia. Anesthesia was induced using thiopentone 5 mg/kg or 4% sevoflurane and 60% nitrous oxide in O2 if venous accessibility could not be obtained before anesthesia induction. Simultaneously, with orotracheal intubation, intravenous warmed to 37°C lactated ringer’s solution at 10 mL/kg/hour was infused. After intubation, anesthesia was maintained with manual assistance of 60% nitrous oxide in O2, 2% sevoflurane, and spontaneous ventilation. Children were covered with warmed surgical sheets over their calves, chest and thighs yet were not actively heated. Ambient temperature was set at 23 - 25°C with constant humidity.
The study drug was administered intravenously after anesthesia induction. Volatile anesthetic agents were discontinued at the end of surgery, and tracheal extubation was done when sufficient spontaneous ventilation (respiratory rate > 12 breaths/minute, VT > 5 mL/kg) was acceded and the children regained cough or gag reflex. The children were brought to the recovery room, after they were fully awake. The shivering intensity was graded using a standardized four-point scale (15) (Table 1) at the following time points: T0, at the recovery room; and T10, 10 minutes; T20, 20 minutes; T30, 30 minutes, thereafter. For inhibiting unwanted complications, clonidine 0.5 μg/kg was prepared as a rescue drug to stop grade 3 and 4 of shivering intensity at T30 in both study groups.
Grading of Shivering Intensity
| Grade | Clinical Signs |
|---|---|
| 0 | No shivering |
| 1 | Peripheral, piloerection vasoconstriction, or both are present but no visible shivering |
| 2 | Muscular activity in only one muscle group |
| 3 | Muscular activity in more than one muscle group but no generalized shivering (moderate muscular activity) |
| 4 | Shivering involving the whole body (violent muscular activity) |
Heart Rate with systolic and diastolic BP was measured before, quickly after and at the 5, 10, 15, 25, and 30 minutes after general anesthesia. Simultaneously with recording shivering intensity, peripheral oxygen saturation, body temperature, and ketamine-related side effects such as hallucinations, nausea, vomiting, blurred vision, urinary retention, hypotension, tachycardia, and seizure were recorded. Intravenous metoclopramide (10 mg) was administered for children with nausea or vomiting, and respiratory depression was supported with active ventilation in both study groups. All children were discharged the day after tonsillectomy. All of the data were recorded by a blinded trained anesthesiology resident.
3.1. Statistical Analysis
In regards to the preliminary study (16) and to reach a power of 90%, for determination of significant differences regarding the shivering intensity within the study groups (P = 0.05, 2-sided), thirty patients were required for each group. Finally, we enrolled 40 children in each group to compensate any refusal or non-valuable data. To determine the sample size, online software was used (please see: http://www.stat.ubc.ca/rollin/stats/size/). The statistical package for social science (SPSS) for windows, version 15 (SPSS Inc., Chicago, IL) was used for data analysis. Data are reported as means ± standard deviation (SD) or median for quantitative variables and percentages for categorical variables. Independent T-test was used to compare results between the groups, paired T-test was used to compare results within groups, and Chi-square sample test was used to compare proportions. A two-sided P < 0.05 was considered statistically significant.
4. Results
Out of the 80 children who were enrolled in the study, no one was lost to follow-up; thus, the number of children who finished the study was eighty. In regards to the demographic and surgical data, no significant differences between the two study groups were seen (Table 2). All baseline characteristics were comparable between both study groups.
| Variable | Group K (n = 40) | Group N (n = 40) | P Value |
|---|---|---|---|
| Age, y | 9.57 (2.92) | 9.97 (6.52) | 0.72 |
| Height, cm | 129.28 (10.51) | 126.76 (12.02) | 0.32 |
| Weight, kg | 27.29 (7.31) | 25.71 (9.01) | 0.39 |
| Heart rate, beats per minute | 92.24 (10.97) | 90.03 (9.46) | 0.33 |
| SBP, mmHg | 118.29 (7.34) | 120.71 (9.27) | 0.19 |
| DBP, mmHg | 76.17 (6.01) | 78.29 (13.14) | 0.35 |
| Peripheral oxygen saturation | 99.01 (0.91) | 98.97 (0.99) | 0.85 |
| Core temperature | 36.97 (0.52) | 37.14 (0.29) | 0.07 |
| Lowest core temperature | 36.34 (0.47) | 36.51 (0.39) | 0.08 |
| Lowest peripheral temperature | 34.17 (0.41) | 34.29 (0.56) | 0.27 |
| Anesthesia duration, minute | 30.01 (3.28) | 31.28 (3.09) | 0.08 |
| Surgery duration, minute | 27.99 (1.02) | 28.54 (1.73) | 0.09 |
As shown in Figure 2, the mean systolic pressure was 119.61 ± 16.79 in the K group and 120.91 ± 13.67 in the N group, before the general anesthesia. Moreover, before anesthesia induction, the mean diastolic pressure was 77.67 ± 6.01 in the K group and 79.77 ± 9.24 in the N group (Figure 3). No arterial hypotension (systolic blood pressure < 90 mm Hg) was reported. According to Figure 4, no tachycardia or bradycardia were reported.
Changes in Mean Systolic Pressure Between Groups K and N
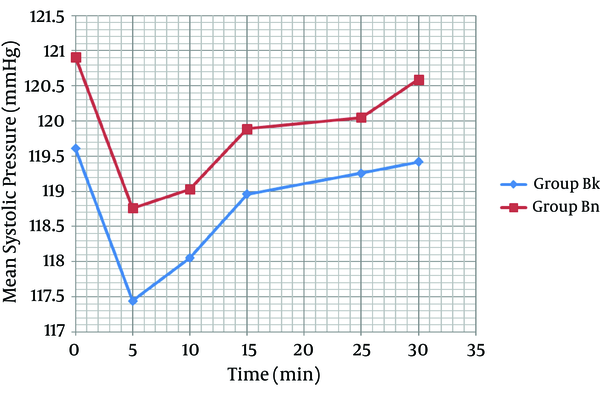
Each bar represents mean ± SD. Case group (n = 40) received general anesthesia that consisted of ketamine 0.5 mg/kg. Control group (n = 40) received general anesthesia that consisted of normal saline. There were no significant differences within and between group K and N before, quickly after and at 5, 10, 15, 25, and 30 minutes after general anesthesia.
Changes in Mean Diastolic Pressure Between Groups K and N
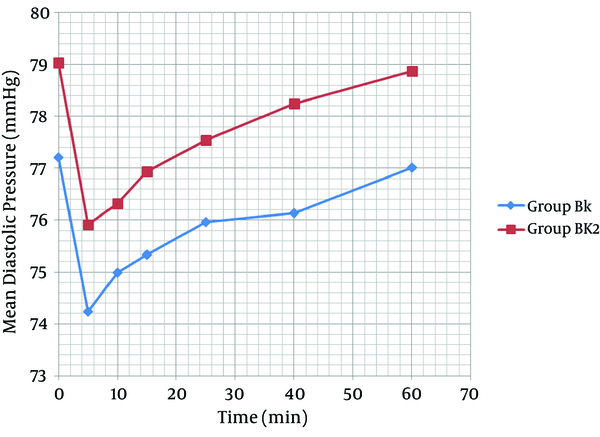
Each bar represents mean ± SD. Group K (n = 40) received general anesthesia that consisted of ketamine 0.5 mg/kg. Group N (n = 40) received general anesthesia that consisted of normal saline. There were no significant differences within and between group K and N before, quickly after and at the 5, 10, 15, 25, and 30 minutes after general anesthesia.
Changes in Heart Rate Between Groups K and N
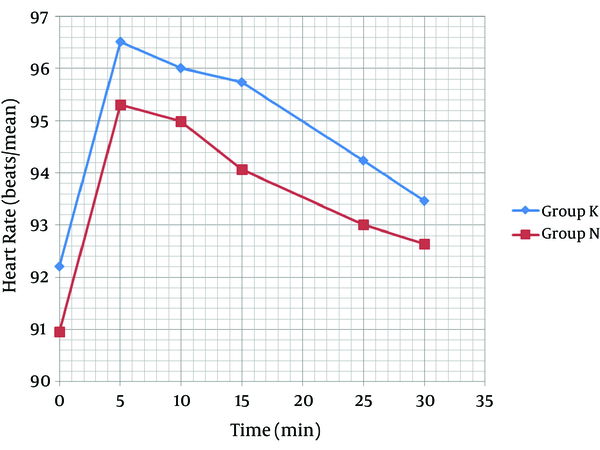
Each bar represents the mean ± SD. Group K (n = 40) received general anesthesia that consisted of ketamine 0.5 mg/kg. Group N (n = 40) received general anesthesia that consisted of normal saline. There were no significant differences within and between group K and N before, quickly after and at the 5, 10, 15, 25, and 30 minutes after general anesthesia.
Shivering intensity in children who had received ketamine was significantly lower than children who did not receive ketamine at the recovery room, and 10, 20, and 30 minutes after arrival (P < 0.05, Table 3). None of the patients in the K group experienced grade 3 of shivering and none of the participants of both study groups had grade 4 of shivering. All of the patients in K group had no shivering in T30 after arrival to the recovery room (Table 3). No significant differences regarding ketamine-related side effects were reported between the two study groups (Table 4, P ≥ 0.05).
Shivering Intensity in Patients of the Ketamine and Placebo Groups on Arrival to the Recovery Room (T0), and Ten (T10), Twenty (T20), and Thirty (T30) Minutes After Arrival
| Time After Arrival To The Recovery Room | Grade 0/1/2/3/4 | P Value | |
|---|---|---|---|
| Group K | Group N | ||
| Number | 40 | 40 | |
| T0 | 38/0/2/0/0 | 11/11/15/3/0 | < 0.0001 |
| T10 | 38/2/0/0/0 | 9/7/20/4/0 | < 0.0001 |
| T20 | 39/1/0/0/0 | 9/7/24/0/0 | < 0.0001 |
| T30 | 40/0/0/0/0 | 11/12/17/0/0 | < 0.0001 |
| Variable | Group K | Group N | P Value |
|---|---|---|---|
| Hallucination | 0 | 0 | NS |
| Nausea | 2 (5) | 1 (2) | NS |
| Vomiting | 1 (2) | 1 (2) | NS |
| Hypotension | 0 | 0 | NS |
| Tachycardia | 0 | 0 | NS |
| Blurred vision | 0 | 0 | NS |
| Seizure | 0 | 0 | NS |
| Urinary retention | 0 | 0 | NS |
5. Discussion
According to the data revealed from the present study, administration of intravenous ketamine at a dosage of 0.5 mg/kg exactly after anesthesia induction has a preventive effect on shivering intensity without hemodynamic alterations in children undergoing general anesthesia for tonsillectomy. Moreover, the use of intravenous ketamine at the doses used in the current study does not significantly increase unwanted side effects such as hallucination, nausea, and vomiting in children.
The data of the current study revealed that intravenous administration of 0.5 mg/kg of ketamine provides effective anti-shivering postoperatively. In consonance to our study, Dal et al. (11) found that ketamine, 0.5 mg/kg intravenously, is effective in prevention of shivering after general anesthesia. In a similar study, Zahra et al. (16) reported that 1 mg/kg of intramuscular ketamine is effective in inhibiting post-operative shivering in children. Kose et al. (12) showed that 0.5 and 0.75 mg/kg of ketamine intravenously is more effective than meperidine 25 mg for prevention and treatment of post-operative shivering in adults and confirmed the results of the current study; however, our study was performed on children. Nevertheless, ketamine’s mechanism of action is not completely understood; it is not clear whether it acts via opioid receptors or directly on the thermoregulatory center (11).
No relationship has been demonstrated between occurrence of shivering and core temperature (17). Furthermore, post-anesthetic shivering can be controlled by pharmacological agents, radiant heat application, and skin surface warming (17). Similar to Kose et al. (12) we found no significant differences in core and peripheral temperature between the study groups. All temperatures of children were over 36ºC and there was no need for active warming. In contrast, Zavareh et al. (18) found a positive significant relationship between body temperature and post-operative shivering. This discrepancy may be related to differences of factors such as temperature of the operation room, duration of surgery, solution infusion, and other active warming methods, which are considered as risk factors for shivering and hypothermia.
However, a low dose of ketamine (0.5 mg/kg) for general anesthesia has a strong effect as an anesthetic agent, yet a higher dose of ketamine (0.75 mg/kg or higher) is associated with hallucination, nausea, vomiting, hemodynamic dysfunction, blurred vision, and seizure in a dose dependent manner (12). In the current study, due to the low dose of ketamine (0.5 mg/kg), hallucination, hemodynamic dysfunction, blurred vision, and seizure did not occur. Furthermore, there were no significant differences in nausea and vomiting between the two study groups.
The large sample size of the studied population is one of the advantages of the current study, which resulted in significant differences between variables and proportions. Furthermore, the data for continuous variables were not dichotomized, which additionally impacted the precision of the analysis. One of the limitations of the present study was the short duration and low blood loss of the procedure (tonsillectomy). In this regard, most of the time significant hypothermia and shivering did not occur.
In conclusion, the data of the current study suggest that an intravenous administration of low dose ketamine in children undergoing general anesthesia inhibits the occurrence of shivering without hemodynamic alterations, and reduces the occurrence of unwanted side effects related to a high dose of intravenous ketamine infusion.
Acknowledgements
References
-
1.
Kolettas A, Lazaridis G, Baka S, Mpoukovinas I, Karavasilis V, Kioumis I, et al. Postoperative pain management. J Thorac Dis. 2015;7(Suppl 1):S62-72. [PubMed ID: 25774311]. https://doi.org/10.3978/j.issn.2072-1439.2015.01.15.
-
2.
Rastegarian A, Ghobadifar MA, Kargar H, Mosallanezhad Z. Intrathecal Meperidine Plus Lidocaine for Prevention of Shivering during Cesarean Section. Korean J Pain. 2013;26(4):379-86. [PubMed ID: 24156005]. https://doi.org/10.3344/kjp.2013.26.4.379.
-
3.
Ghobadifar MA, Zabetian H, Karami MY. Spinal pethidine and shivering for elective caesarean section. Anaesth Intensive Care. 2013;41(6):805. [PubMed ID: 24180725].
-
4.
Schafer M, Kunitz O. Postoperative shivering. Anaesthesist. 2002;51(9):768-84. [PubMed ID: 12232650]. https://doi.org/10.1007/s00101-002-0381-y.
-
5.
Ghobadifar MA, Zabetian H, Karami MY, Mosallanezhad Z, Kalani N. Importance of maternal body temperature recording after injection of meperidine during spinal anesthesia in patients undergoing cesarean section: an offering for conducting clinical studies. Rev Bras Anestesiol. 2014;64(6):449. [PubMed ID: 25437707]. https://doi.org/10.1016/j.bjan.2014.02.004.
-
6.
Faiz SH, Rahimzadeh P, Imani F, Bakhtiari A. Intrathecal injection of magnesium sulfate: shivering prevention during cesarean section: a randomized, double-blinded, controlled study. Korean J Anesthesiol. 2013;65(4):293-8. [PubMed ID: 24228140]. https://doi.org/10.4097/kjae.2013.65.4.293.
-
7.
Imani F, Rahimzadeh P. Gabapentinoids: gabapentin and pregabalin for postoperative pain management. Anesth Pain Med. 2012;2(2):52-3. [PubMed ID: 24223337]. https://doi.org/10.5812/aapm.7743.
-
8.
Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on N-methyl-d-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44(3):293-9. https://doi.org/10.1016/0304-3959(91)90100-c.
-
9.
Woolf CJ, Chong M. Preemptive Analgesia—Treating Postoperative Pain by Preventing the Establishment of Central Sensitization. Anesthesia & Analgesia. 1993;77(2):362-79. https://doi.org/10.1213/00000539-199377020-00026.
-
10.
Javid MJ, Hajijafari M, Hajipour A, Makarem J, Khazaeipour Z. Evaluation of a low dose ketamine in post tonsillectomy pain relief: a randomized trial comparing intravenous and subcutaneous ketamine in pediatrics. Anesth Pain Med. 2012;2(2):85-9. [PubMed ID: 24223344]. https://doi.org/10.5812/aapm.4399.
-
11.
Dal D, Kose A, Honca M, Akinci SB, Basgul E, Aypar U. Efficacy of prophylactic ketamine in preventing postoperative shivering. Br J Anaesth. 2005;95(2):189-92. [PubMed ID: 15849207]. https://doi.org/10.1093/bja/aei148.
-
12.
Kose EA, Dal D, Akinci SB, Saricaoglu F, Aypar U. The efficacy of ketamine for the treatment of postoperative shivering. Anesth Analg. 2008;106(1):120-2. table of contents. [PubMed ID: 18165565]. https://doi.org/10.1213/01.ane.0000296458.16313.7c.
-
13.
Eydi M, Golzari SE, Aghamohammadi D, Kolahdouzan K, Safari S, Ostadi Z. Postoperative Management of Shivering: A Comparison of Pethidine vs. Ketamine. Anesth Pain Med. 2014;4(2). eee15499. [PubMed ID: 24829883]. https://doi.org/10.5812/aapm.15499.
-
14.
Park SM, Mangat HS, Berger K, Rosengart AJ. Efficacy spectrum of antishivering medications: meta-analysis of randomized controlled trials. Crit Care Med. 2012;40(11):3070-82. [PubMed ID: 22890247]. https://doi.org/10.1097/CCM.0b013e31825b931e.
-
15.
Crossley AW, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia. 1994;49(3):205-7. [PubMed ID: 8147511].
-
16.
Zahra FA, Abudallah HM, Shabana RI, Abdulmageed WM, Abdulrazik SI, Nassar AM. Intramuscular ketamine for prevention of postanesthesia shivering in children. Saudi Med J. 2008;29(9):1255-9. [PubMed ID: 18813407].
-
17.
Vanderstappen I, Vandermeersch E, Vanacker B, Mattheussen M, Herijgers P, Van Aken H. The effect of prophylactic clonidine on postoperative shivering. A large prospective double-blind study. Anaesthesia. 1996;51(4):351-5. [PubMed ID: 8686824].
-
18.
Zavareh SMHT, Morovati L, Koushki AM. A comparative study on the prophylactic effects of ketamine, dexamethasone, and pethidine in preventing postoperative shivering. J Res Med Sci. 2012;17.