1. Background
Successful spinal anesthesia administration is crucial to surgical procedures. Spinal anesthesia procedures have a 83% - 99% success rate (1). Spinal anesthesia success rate is affected by several factors, such as the quality of the injection landmark, quality radiologic images of the vertebrae, the skill of the anesthesiologist, patient position, lumbar flexion, and the distance between the skin and the subarachnoid space (2-5). Spinal anesthesia has also proven to be advantageous over general anesthesia, as seen in urology surgeries such as percutaneous nephrolithotomy and trans-urethral resection of the prostate. Documented advantages include better maintenance of hemodynamic state, decreased analgesic need, shorter surgery duration, reduced deep vein thrombosis rate, and fewer general anesthesia adverse effects (6-8).
Patient position during spinal anesthesia administration determines whether the insertion of the spinal needle into the subarachnoid space is successful or not. Poor positioning may cause repeated spinal needle insertions and spinal needle-vertebrae bone contact, thus increasing the risk of back pain, post-dural puncture headache (PDPH), epidural hematoma, and neural trauma (9).
There are two common positions used for spinal needle insertion: the sitting position and the lateral decubitus position. Each position has its own advantages. In the sitting position, it is easier to identify the midline, especially in obese patients and patients with vertebral bone diseases (i.e., scoliosis). In the lateral decubitus position, the patient’s position is more stable, especially in sedated patients or in those who have difficulty maintaining their body position. More than 90% of spinal anesthesia procedures performed in the central national hospital Dr. Cipto Mangunkusumo used the sitting position.
The main purpose of the sitting position is to optimize lumbar flexion, resulting in easier access to the spinal needle insertion target, which is located in between two spinous processes. Lumbar flexion also pushes the thecal sac into a more superficial position. The sitting position has several variants, such as the traditional sitting position (TSP), the hamstring position, the squatting position, and the pendant position, each of which has its own advantages (10, 11).
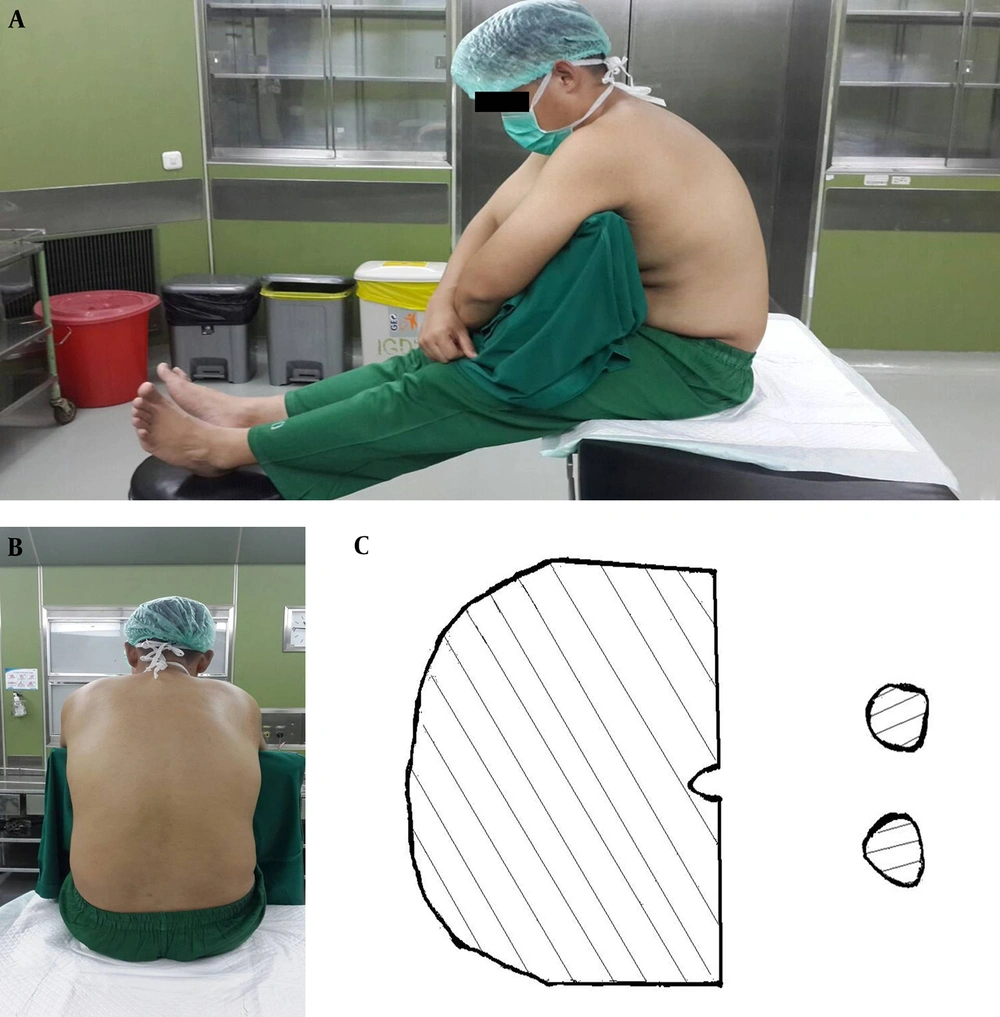
The traditional sitting position is a comfortable position for patients and for the spinal anesthesia operator. The patient sits on the side of the bed, with his or her feet propped up on a chair, and hugs a pillow. The spinal anesthesia operator then has direct access to the median area of the vertebrae without being restricted by the upper part of the bed. This position is the most commonly used variant of the sitting position (12-14). The problem with this position is the need for a chair to prop up the patient’s feet and the need for two assistants to help move the patient’s feet to a supine position. In the TSP, the success rate of spinal needle placement on the first try is 40% - 50% (13). Illustrations of the TSP are found in Figure 1.
The picture was taken from Fisher KS, Arnholt AT, Douglas ME, Vandiver SL, and Nguyen DH. A randomized trial of the TSP versus the hamstring stretch position for labor epidural needle placement. Anesth Analg. 2009; 109: 532 - 4. This picture depicts a model in the TSP. A, the lateral view of the TSP; B, midline shown in the TSP; C, sectional areas holding the body in the TSP.
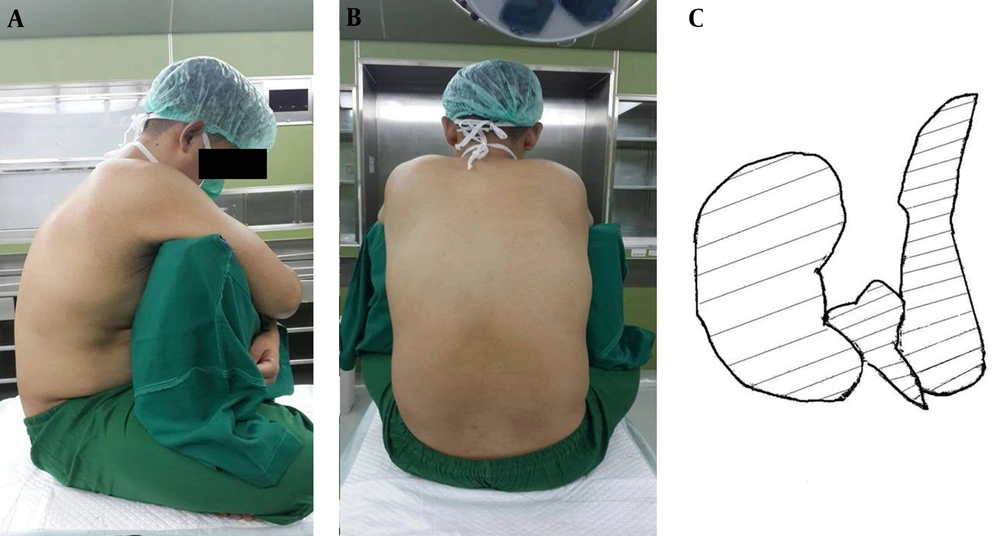
The crossed-leg sitting position (CLSP) is commonly seen in Asia. This position is easy and comfortable for patients. The CLSP causes knee and hip flexion, resulting in posterior pelvic leaning and reduced lumbar lordosis (15, 16). Redai and Flood also state that the CLSP increased lumbar flexion by 10 - 15 degrees compared to the TSP. Optimal vertebrae flexion offers more access to the interspinous and interlamina gap, thus facilitating spinal needle placement in the subarachnoid space (17). Illustrations of the CLSP can be found in Figure 2.
There have been no previous studies about the spinal needle placement success rate in the CLSP. This position can be an alternative position to the spinal sitting position variants based on the rationales explained above.
2. Objectives
The main aim of this study was to compare successful spinal needle placement in the subarachnoid space in the CLSP and the TSP in patients undergoing urology surgery.
3. Methods
This study was a non-blinded, randomized clinical trial performed in the urology operating theatres of central national hospital Dr. Cipto Mangunkusumo in Jakarta, Indonesia in March-October 2015. Approval from the ethical committee of the faculty of medicine Universitas Indonesia was acquired prior to conducting the study. Subjects gave informed consent before enrolling in the study.
Sample size number was calculated according to the unpaired categorical analytical sample size formula for different proportions (18). The pilot study performed at the central national hospital Dr. Cipto Mangunkusumo in Jakarta, Indonesia in May, 2014 stated that the proportion value of patients who underwent spinal anesthesia in the TSP without any bone-needle contacts was 40%. The expected proportion difference was 20%; thus, the minimal sample size needed was 105 subjects for each group, with 5% error type I and 80% power.
Consecutive sampling was used. Interventions were administered to patients in either the CLSP or the TSP. Allocation for interventions was performed using block randomization. Allocations were then concealed in a thick, opaque envelope. Block randomization and concealing were performed by third parties (anesthesiology residents) who were not involved in this study.
The following patients were included: those aged 18 - 60 years and those with ASA physical status I - III who planned to undergo urology surgery with spinal anesthesia. Subjects were informed about the study, agreed to enroll, and signed informed consent forms. The following patients were excluded: uncooperative subjects, subjects with relative and absolute contraindications to spinal anesthesia (coagulation disorders, thrombocytopenia, elevated intracranial pressure, severe hypovolemia, severe heart valve disorders, local infection at the injection site, allergy to local anesthetic agents, significant anatomical disorder of the spine, wound/scar on the lumbar area), and subjects with a body mass index (BMI) > 32 kg/m2 (4). Drop out criteria were as follows: subject requested to drop out of the study, subject was in need of emergency treatment during the spinal anesthesia procedure, and the spinal needle required redirection more than nine times (failed spinal anesthesia procedure).
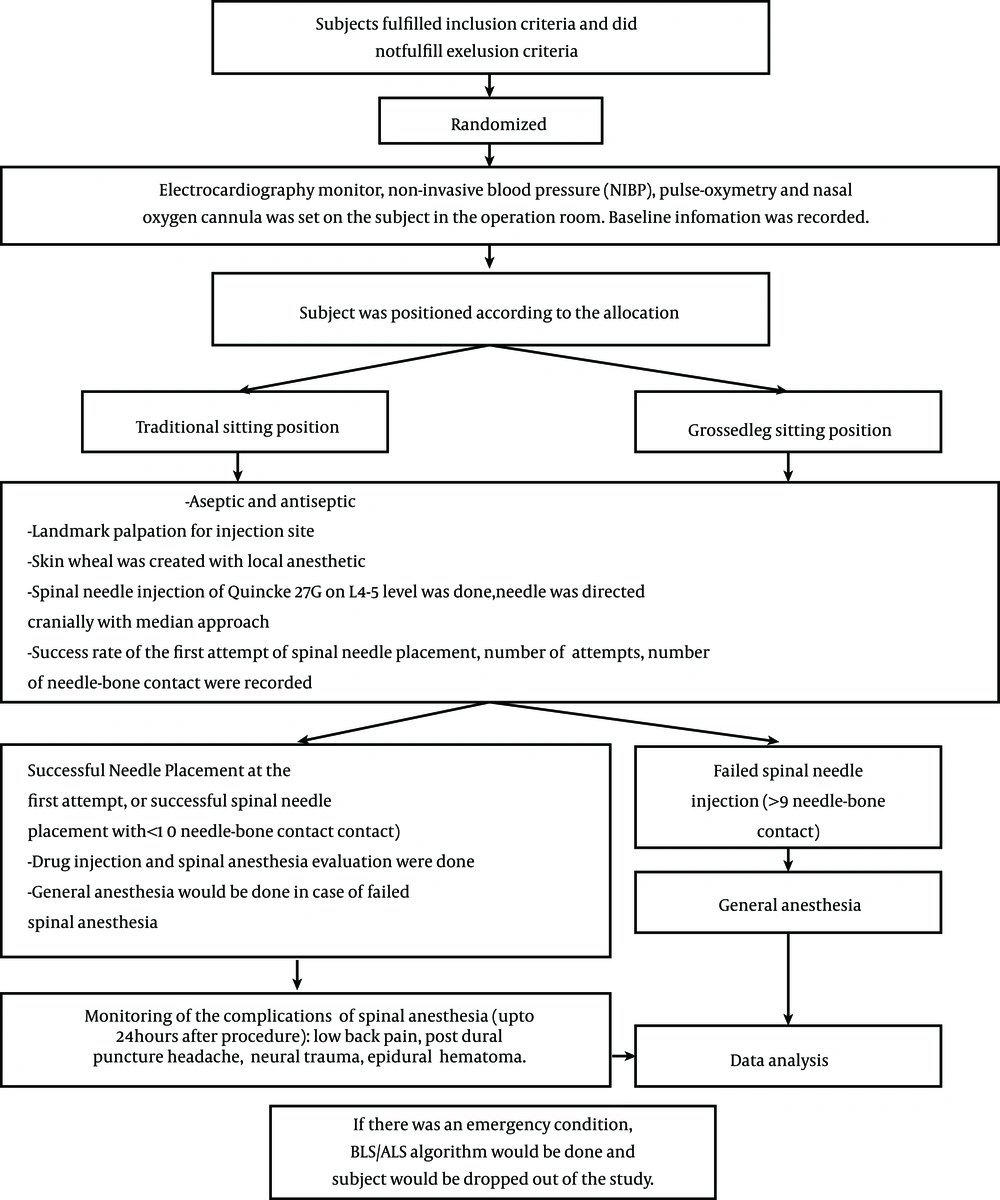
Baseline information for each subject that received allocated intervention was recorded. Surgical procedures and spinal anesthesia procedures were carried out according to the regulations of the urology operating theatres of the central national hospital Dr. Cipto Mangunkusumo. Spinal anesthesia was performed by anesthesiology residents who had performed at least 50 spinal anesthesia procedures and had been informed about the protocol for spinal anesthesia injection in this study (to avoid bias). The following data were recorded for each intervention: the number of successful first attempts of spinal needle placement, the difficulty level of landmark palpation for injection, and the number of needle-bone contacts. The study protocol is shown in Figure 3. Difficulty levels of landmark palpation for injection were classified as follows: easily palpable (the lower border of the superior spinal process and the upper border of the inferior spinal process were clearly palpable, and the second palpation gave a consistent result), hardly palpable (the lower border of the superior spinal process and the upper border of the inferior spinal process were palpable, but the second palpation gave an inconsistent result), and impalpable (the spinal process could not be palpated due to vertebrae malformation, such as scoliosis, presence of a vertebral implant, thick subcutis tissue, or ankylosing spondylitis).
Data was analyzed by SPSS (statistical package for social scientist) 15.0. The characteristics and demographic data of each group are presented descriptively in terms of percentage, mean, and standard deviation. Categorical data was analyzed by chi-square test or Fisher’s exact test. Ordinal data was analyzed by chi-square test or by the Kolmogorov-Smirnov test. The significance value used was 5% with 80% power.
4. Results
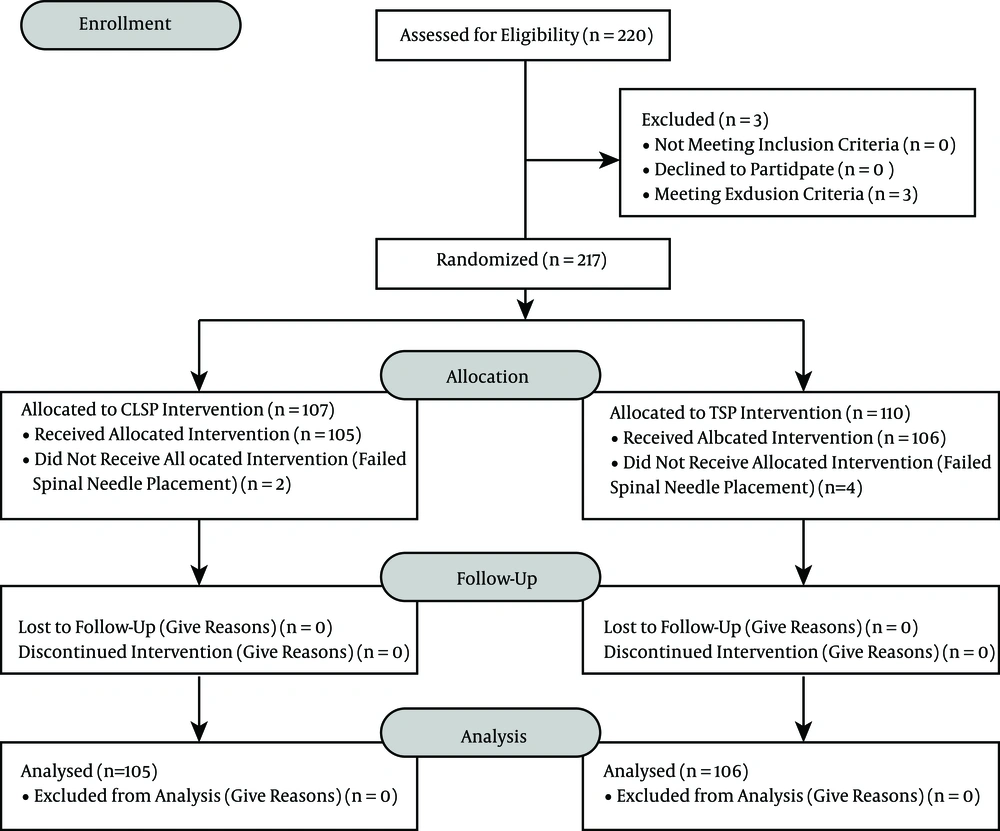
The 220 subjects that were chosen through consecutive sampling fulfilled the inclusion criteria and gave their consent. Three patients were excluded due to their body mass index (> 32kg/m2); thus, 217 subjects were allocated into two groups: the crossed-leg sitting position (CLSP) group (n = 107) and the TSP group (n = 110). Six subjects were dropped out due to failed spinal needle placement (Figure 4). A total of 211 subjects finished the study and were analyzed (Figure 4).
Subject characteristics, including age, sex, BMI, and physical status according to the ASA (American society of anesthesiologists), are shown in Table 1. Both groups had homogenous characteristics; thus, both groups were comparable.
| Crossed-Leg Sitting Position (n = 107) | Traditional Sitting Position (n = 110) | |
|---|---|---|
| Sex, % | ||
| Male | 59 (56.2) | 64 (60.4) |
| Female | 46 (43.8) | 42 (39.6) |
| Age, y | 48.8 (8.6) | 45.2 (9.3) |
| Body Weight, kg | 57.4 (9.8) | 55.4 (8.7) |
| Body Height, cm | 156.6 (6.9) | 159.0 (7.7) |
| BMI, kg/m2 | 22.8 (3.2) | 23 (3.4) |
| ASA Physical status, % | ||
| I | 5 (4.8) | 6 (5.7) |
| II | 93 (88.6) | 97 (91.5) |
| III | 7 (6.6) | 3 (2.8) |
| Operators’ characteristics | ||
| First year (beginner) | 19 | 21 |
| Second year (intermediate) | 48 | 44 |
| Third and fourth year (advanced) | 38 | 41 |
aValues are expressed as mean (SD).
Table 2 shows that the success rate of first-try spinal needle placement in the CLSP group was 62.9%, whereas in the TSP group it was 55.7%. Although the CLSP group showed a higher success rate for spinal needle placement than the TSP group, the difference was not significant (P > 0.05).
| Successful Spinal Needle Placement in One Trya | Number of Needle-Bone Contactsb | Ease of Landmark Palpationc | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | 0 | 1 - 3 | 4 - 9 | Easily Palpable | Hardly Palpable/ Impalpable | ||
| CLSP | 66 (62.9) | 39 (37.1) | 66 (62.9) | 27 (24.5) | 12 (11.4) | 91 (86.7) | 14 (13.3) | 105 (49.8) |
| TSP | 59 (55.7) | 47 (44.3) | 59 (55.7) | 36 (33.9) | 11 (10.4) | 81 (76.4) | 25 (23.6) | 106 (50.2) |
| Total | 125 (59.2) | 86 (40.8) | 125 (59.2) | 63 (29.9) | 23 (10.9) | 172 (81.5) | 39 (18.5) | |
aChi-square test, P = 0.328.
bChi-square test, P = 0.42.
cChi-square test, P = 0.075, P value is significant when P < 0.05.
The ease of landmark palpation was categorized as “easily palpable” or “hardly palpable/impalpable.” In the CLSP group, 86.7% of patients were “easily palpable,” whereas 76.4% of the TSP group was “easily palpable.” There was no significant difference between the groups in terms of ease of landmark palpation (P > 0.05).
The number of needle-bone contacts in both groups was categorized as follows: no needle-bone contacts (“0”), one to three needle-bone contacts (“1 - 3”), and four to nine needle-bone contacts (“4 - 9”) (Table 2). Statistical analysis showed no significant correlation between sitting position and number of needle-bone contacts (P > 0.05).
Table 3 shows that four subjects (3%) suffered from PDPH as a spinal anesthesia complication. Low-back pain after injection occurred in two subjects. There was no neural trauma recorded in this study. Failure or physiological side effects of spinal anesthesia due to anesthetic drugs were not assessed.
| Complication | CLSP Group | TSP Group |
|---|---|---|
| PDPH | 1 (0.9) | 3 (2.9) |
| Low-back pain | 1 (0.9) | 1 (0.9) |
| Neural trauma | 0 | 0 |
aValues are expressed as No. (%).
5. Discussion
Achievement of optimal lumbar flexion is the main goal of spinal needle placement in the subarachnoid space. Lumbar flexion offers access to the interspinous gap and pushes the medulla spinalis to a more superficial position towards the midline skin (13, 19, 20).
The variants of sitting position studied were the CLSP and the TSP. Both sitting positions accomplish lumbar flexion. Biswas et al. (21) stated that having the patient sit with a straightly-aligned back increases the number of spinal needle redirections compared to having the patient sit with a flexed back. In this study, the researchers found that a CLSP caused hip and knee flexion, resulting in posterior pelvic leaning, reduction of lumbar lordosis, and increased lumbar flexion by 10 - 15 degrees compared to the TSP (15-17).
Table 1 shows that 211 subjects in this study had homogenous characteristics. The numbers of male and female subjects were quite balanced. BMI in both groups was classified as normal and thus comparable. The ASA physical statuses of both groups were homogenous, most of which was ASA physical status II. Spinal anesthesia operator levels were spread out evenly in both groups.
Data analysis showed that there was no significant difference in terms of first-time success rate of spinal needle placement between the CLSP group and the TSP group (Table 2). The CLSP group (62.9%) had a higher first-time spinal needle placement success rate than the TSP group (55.7%). The ease of landmark palpation was not significantly different between groups. In the easily palpable category, the result was different by 10 subjects (10.2%). Furthermore, the needle-bone contact number in all categories showed no significant difference. Several factors might affect these results.
The results of this study indicate that 10 - 15 degrees of lumbar flexion (17), which was one of the advantages offered by CLSP, might not adequately increase the opening of the interspinous and interlamina gaps. Adequately opened gaps are very important in the process of spinal needle placement into the subarachnoid space. Moreover, there are several factors regarding the postural differences between the CLSP and the TSP that were not considered in this study. The TSP and the CLSP each has a specific three-dimensional configuration of the vertebrae that can only be evaluated with radiological imaging (11). Thigh adduction and hanging feet position, with the patient propped up by a chair, is typical for the TSP (22). Thigh abduction and crossed legs with each sole of the feet under the contralateral thigh is typical for the CLSP. A three-dimensional CT scan could be used to evaluate these specific configurations (12, 13, 15, 16, 23).
Needle type also affects successful spinal needle insertion. Rand et al. stated that use of a Quincke needle created greater deflection compared to that of a Whitacre needle; thus, the Quincke needle might not be as reliable as the Whitacre needle. In this study, a Quincke needle was used. Further studies using Whitacre needles or another needle type with a wider diameter are needed (24).
Pryambodho et al. (14) compared spinal needle placement success rates between the pendant position and the TSP in 2014, and they found that the pendant position had a higher success rate of spinal needle placement. Pendant position is a sitting position with the patient’s underarms propped up by a cantilever. Propped underarms reduce vertical pressure (gravity) on the vertebrae, thus increasing intervertebral distance and interspinous and interlamina gap distance. In this study, subjects were not propped up; they hugged a pillow instead. The pillow was used to increase and maintain lumbar flexion. We assume, a cantilever could be used as a replacement for the pillow to increase the distance of the interspinous and interlamina gaps by reducing vertical pressure between vertebrae bodies in addition to increasing lumbar flexion. However, no studies have been performed using a cantilever as a factor to increase intervertebral distance.
The CLSP was expected to create more hip flexion than the TSP. Hip flexion pushes the lumbar vertebrae to the posterior side, shortening the distance between the spinal processes and the skin, which may aid identification of the spinous processes as landmarks. There were no significant differences between the groups in terms of ease of landmark palpation. This might be caused by inadequate difference in lumbar vertebrae furtherance in both groups (15, 16). Many studies have found landmark identification to be one of the important factors for successful spinal anesthesia administration, although its accuracy in evaluating the required intervertebral gaps is poor (1, 4, 9, 25, 26).
Another advantage of the CLSP is patient comfort; the CLSP provides a larger surface area for the legs to hold the body compared to the TSP, as shown in Figure 2C. The larger surface area to hold the body towards the lateral sides also provides stability to maintain the patient’s sitting posture. Moreover, the CLSP showed lower abdominal muscle activity compared to the TSP; thus, patients can maintain their body positions more easily in the CLSP than in the TSP (27). In certain populations, such as patients with a BMI > 32 kg/m2, geriatrics (patients older than 60 years) and patients without back pain, the CLSP may increase the first try success rate of spinal needle placement ease landmark identification, and reduce the number of needle-bone contacts. On the other hand, the CLSP increases pressure in the intervertebral discs, resulting in the worsening of low back pain in patients with herniated nucleus pulposus (HNP) (15).
PDPH occurred in four subjects (Table 3). Headaches reported by subjects were localized in the frontal or occipital area, pulsating, and increased in intensity when subjects sat up or stood up. The VAS (visual analog scale) value was 2 - 4. PDPH was managed by the analgesic drug paracetamol, bed rest, and intravenous hydration. All four subjects improved after treatment, and no PDPH was reported when subjects were discharged.
The incidence of PDPH after spinal anesthesia administration is 2.5% - 9.3% (28-30). PDPH risk factors include female gender, age 31 - 50 years old, history of PDPH, perpendicular bevel orientation and pregnancy (29). In this study, the four subjects that suffered from PDPH were non-pregnant women in their fourth or fifth decades of life.
Back pain after spinal anesthesia occurred in two subjects. The pain was localized in the injection area with VAS 1-3. Neither subject received analgesic therapy apart from that administered for the surgery because they did not experience a sufficient degree of pain. There was no pain reported when the subjects were discharged. The characteristics of the pain reported in this study were similar to those reported in Chan’s study in 1995: localized to the injection area as a pain on palpation (31). There was no difference in back pain incidence or PDPH incidence between the groups; thus, it can be concluded that CLSP did not increase or decrease the risk of spinal anesthesia complications compared to TSP.
This study had several limitations. Blinding was not possible due to the apparent differences between the interventions. In addition, the parameters measured were subjective. Further studies should be performed using devices (e.g., calipers, ultrasonography, radiographic tools, etc.) to measure the lumbar angulation degree, the interspinous gap distance, the furtherance of spinal processes to the skin (superficial furtherance), and the accuracy of evaluation of the intervertebral gap.
5.1. Conclusion
The success rate of spinal needle placement in the CLSP group was not significantly different from that in the TSP group; thus, CLSP can be used as an alternative sitting position in spinal anesthesia procedures.
5.2. Suggestions
Further studies to compare the success rate of spinal needle placement between the CLSP and the TSP in certain populations specifically subjects with higher than normal BMI and geriatrics should be performed. In addition, further studies to investigate postural differences between the CLSP and the TSP should be performed by objective measurements (vertebral angulation degree should be measured by pelvic radiologic imaging, and interspinous gap distance should be measured by caliper or ultrasonography). To minimalize needle deflection, a pencil-point needle and/or a needle with a wide diameter should be used.