Abstract
Background:
Caudal analgesia is a common method for postoperative pain management in pediatric patients. Additive agents such as opioids and α2 agonists have been used to enhance the analgesic effects of local anesthetics for caudal block.Objectives:
The aim of this study was to compare the additive effects of dexmedetomidine and fentanyl on bupivacaine-induced caudal analgesia in pediatric patients who had undergone elective inguinal hernia repair.Methods:
This randomized, double-blind clinical trial included children aged 1 - 5 years who were divided into three groups: the bupivacaine group (Group B) received 0.25% bupivacaine (1 ml/kg), the bupivacaine-dexmedetomidine group (Group BD) received 0.25% bupivacaine (1 mL/kg) plus 2 µg/kg dexmedetomidine, and the bupivacaine-fentanyl group (Group BF) received 0.25% bupivacaine (1 mL/kg) plus 2 µg/kg fentanyl. The hemodynamic variables (heart rate, systolic blood pressure, respiratory rate, and peripheral arterial oxygen saturation) were measured perioperatively. Pain, sedation and motor block scores and adverse events (nausea and vomiting, pruritis, hypotension, bradycardia, urinary retention and respiratory depression) were documented at 30 and 60 minutes, and the 1st, 2nd, 4th, 6th, 12th and 24th hours after the operation. The other recordings include the duration of surgery and analgesic requirement.Results:
A total of 61 patients were analyzed. The lowest pain scores were found in the BD group at all time points (P < 0.001). The sedation scores were higher in the BD group than in the other two groups at all time points (P < 0.001). No motor block was observed after the operation. Only three patients required analgesic administration 2 to 6 hours after the operation in group B. No side effects were observed in any of the groups, and there was no significant difference in the duration of surgery among the three groups.Conclusions:
The results show that the analgesic and sedative effects were better when dexmedetomidine was added to bupivacaine than when fentanyl was added or bupivacaine alone was administered in the pediatric population studied here that underwent elective inguinal hernia repair.Keywords
Anesthesia Caudal Bupivacaine Dexmedetomidine Fentanyl Postoperative Pain
1. Background
If acute pain is left untreated or not treated properly, it can progress to chronic pain. There is a large amount of evidence for the use of multi-modal approaches to counteract pain in the pediatric population (1). Caudal analgesia has been widely used as a pain management modality in a variety of pediatric operations (2). Bupivacaine is a long-acting reliable local anesthetic agent that is used as a caudal analgesic, but different auxiliary agents need to be co-administered to improve its analgesic efficient (3, 4). Single shots of a combination of local anesthetics, such as ketamine, midazolam, neostigmine, adrenaline, opioids, clonidine and, recently, dexmedetomidine, have been used (5). Opioids are effective analgesic agents, but they are associated with respiratory depression, itching, urinary bladder dysfunction, nausea and vomiting (6). Clonidine and dexmedetomidine are both α2 adrenergic receptor agonists, but dexmedetomidine has eight times stronger receptor affinity than clonidine. Dexmedetomidine has sedative, hypnotic, anxiolytic, analgesic, anesthetic-sparing and sympatholytic effects and does not have any adverse effects on respiratory or cardiovascular functions (7).
2. Objectives
The aim of this study was to compare the postoperative analgesic effects of bupivacaine alone with the additive effects of fentanyl and dexmedetomidine on bupivacaine-induced caudal analgesia.
3. Methods
This randomized, double-blind clinical trial included children aged 1 to 5 years who were admitted to the operating room for elective inguinal hernia repair. According to the tenets of the Declaration of Helsinki, the written informed consent of their parents was obtained prior to the intervention.
One-sided analysis of variance of the mean values from a previous study was used to calculate the sample size for the present study (8), with α = 0.05, β = 0.1, σ = √MSE = 1/1, µ1 = 2.5, µ2 = 2.5, and µ3 = 1.25. The calculated λ resulted in a non-central distribution with χ2 equal to 12.66 and the number of cases in each group (Δ) equal to 16. The number of patients who were likely to drop out was calculated to be 20 in each group (NCSS software).
The study was a double-blind randomized one (Random Allocation Software, version 1.0.0). A total of 81 patients were enrolled in the study and 20 were excluded (the flow chart for patient selection is shown in Figure 1). Children with cardiopulmonary congenital anomalies, a history of drug allergy to the drugs used, contraindications for caudal block, and ASA class > I were excluded.
CONSORT Flow Diagram
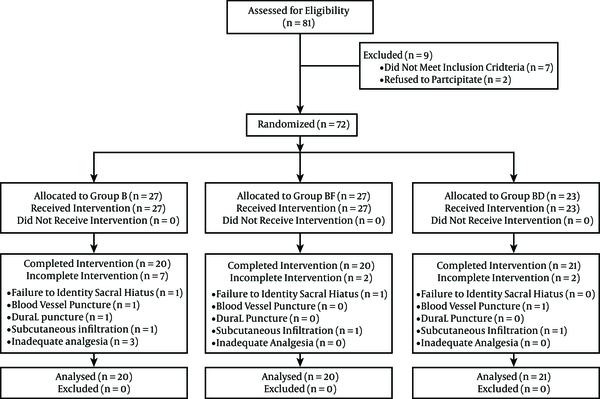
No premedication was administered to the children. Anesthesia was induced through inhalation of 50% oxygen/50% nitrous oxide and sevoflurane (7% every four successive breaths). This was followed by intravenous administration of atracurium (0.5 mg/kg) and endotracheal intubation. The child was placed in the lateral position, and the caudal area was prepared and sterilized. A 23-gauge needle was inserted in the sacral hiatus area by an experienced anesthesiologist. The children were randomly assigned to one of the following groups: the bupivacaine group (Group B), in which 1 mL/kg of 0.25% bupivacaine was administered (Marcaine Spinal 0.5% Heavy, AstraZeneca, Horizon Place, 600 Capability Green, Luton, Bedfordshire, LU1 3LU); the bupivacaine-dexmedetomidine group (Group BD), in which 1 mL/kg of 0.25% bupivacaine and 2 µg/kg dexmedetomidine were administered (Precedex®; Hospira, Lake Forest, Illinois, USA); and the bupivacaine-fentanyl group (Group BF), in which 1 mL/kg of 0.25% bupivacaine and 2 µg/kg fentanyl were administered (fentanyl citrate; Caspian Tamin Pharmaceutical Co., Rasht-Iran). All solutions were prepared from 0.5% bupivacaine to which distilled water was added to prepare the 0.25% solution. Anesthesia was maintained with sevoflurane (MAC 2.0% - 2.6%, age adjusted) throughout the operation. Data were collected by an anesthesiology resident, who was blinded to the type of caudal analgesic solution administered. The hemodynamic indices (heart rate (HR) and systolic blood pressure) were maintained in the 20% pre-induction range. If the value of the indices exceeded this range, the analgesics administered were considered to be insufficient and an intravenous dose of 1 µg/kg fentanyl was administered. Moreover, the patient was excluded from the study. The hemodynamic variables (HR, systolic blood pressure (SBP), respiratory rate, and peripheral arterial oxygen saturation) were measured before and every 5, 10 and 15 minutes after the analgesics were administered; every 5 minutes during the operation; and every 15 minutes in the recovery room. Ringer’s solution was administered at a dosage of 4 - 6 mL × kg-1 × h-1 perioperatively. On completion of the surgery and reversal of the effects of the neuromuscular blocking agents, the patients were evaluated in the recovery room and surgical ward (for 24 hours) by the anesthesiology resident in charge of the study. The scores for pain, sedation and motor block were determined at 30 and 60 minutes and the 1st, 2nd, 4th, 6th, 12th and 24th h after the operation.
The Persian version of the face pain scale-revised (FPS-R) tool, scored on a scale of 0, 2, 4, 6, 8, and 10, was used to measure pain (9). If the pain score was equal to or more than 4, a rescue analgesic agent (meperidine, 1 mg/kg) was administered.
The level of sedation was measured at the same time points as mentioned above with the University of Michigan sedation scale (UMSS) (0 = awake and conscious, 1 = mild sedation, 2 = moderate sedation, 3 = deep sedation, 4 = no response) (10).
The level of motor block was assessed by the modified Bromage score (MBS) with 0 = free movement, 1 = only able to bend the knees and move the feet, 2 = unable to bend the knees but moves feet, and 3 = unable to move the feet (11).
The patients were also assessed at the above time points for adverse events such as nausea and vomiting, pruritis, hypotension (SBP < 70 + age × 2), bradycardia (HR < 100/min for 1-year-old patients, and HR < 95/minutes for 2- to 5-year-old patients), urinary retention (inability to voluntarily empty the bladder for more than 12 hours, with urine volume greater than that expected for the patient age ((age in years + 2) × 30 cc), or a palpably distended bladder) and respiratory depression (respiratory rate less than 10). The postoperative recordings included the duration of surgery, time of first analgesia administration, and occurrence and treatment of postoperative nausea and vomiting (PONV) and pruritus. PONV and pruritis were treated by intravenous administration of 0.1 mg/kg ondansetron in the form of a slow bolus injection.
3.1. Statistical Analysis
The Kolmogorov-Smirnov Z test was used to analyze the equality of the distributive functions of the variables. A one-way analysis of variance (ANOVA) and a generalized linear model repeated-measure ANOVA were used to analyze the parametric variables. For non-parametric variables (pain sedation scores and duration of surgery), the Kruskal-Wallis test, Friedman Test and chi-square test were applied. Later, Bonferroni post-hoc analysis was used to analyze the difference between groups. P values < 0.05 were considered to indicate statistical significance.
3.2. Ethics Statement
This study was approved by the deputy for research and medical ethics committee of Hormozgan University of Medical Sciences (4-HEC-93-7-8) and registered with the Iranian registry of clinical trials (IRCT2015110318091N6).
4. Results
A total of 61 patients were analyzed, of whom 56 (91.8%) were boys and 5 (8.2%) were girls. However, no significant relationship was found between gender and the groups in the study. The other demographic data (age, height and weight) were not significantly different between the groups (Table 1).
| Group B | Group BD | Group BF | Degree of Freedom | P Value | |
|---|---|---|---|---|---|
| ASA I/II | 18/2 | 0/20 | 0/21 | - | 0.12 |
| No. of boys | 20 | 18 | 18 | 2 | 0.23 |
| No. of girls | 0 | 2 | 3 | ||
| Age, mo | 24 | 36 | 36 | 46 | 0.21 |
| Height, cm | 84.05 ± 13.61 | 92.80 ± 12.82 | 87.00 ± 10.97 | 2 | 0.09 |
| Weight, kg | 10.80 ± 3.03 | 12.15 ± 2.34 | 12.95 ± 3.77 | 3 | 0.09 |
| Duration of surgery, min | 33.70 ± 12.98 | 33.00 ± 11.96 | 41.65 ± 11.79 | F test 8.685 | 0.054 |
4.1. Pain
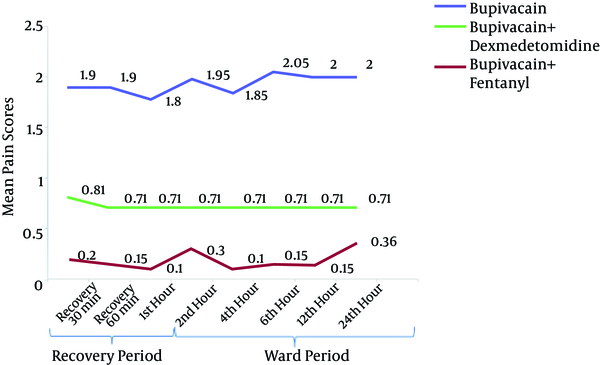
There was no significant difference in the mean postoperative pain scores between different time points in each group. However, the mean pain scores were significantly different between the three groups at all time points (P < 0.001), with the subsequent Bonferroni test indicating a significant difference between group B and BD (P < 0.001) and between group B and BF (P < 0.001). The lowest mean scores were observed in the BD group. In the 1st, 2nd and 4th h after the operation, the mean pain scores were significantly lower in the BD group than in the BF group (P < 0.001). However, in the following hours (6th, 12th and 24th), the mean pain scores in the BD group were lower but not significantly different from those in the BF group (Figure 2).
Pain Scores of the Three Groups (Bupivacaine, Bupivacaine-Dexmedetomidine and Bupivacaine-Fentanyl) After the Operation (Recovery and Ward Period)
4.2. Sedation
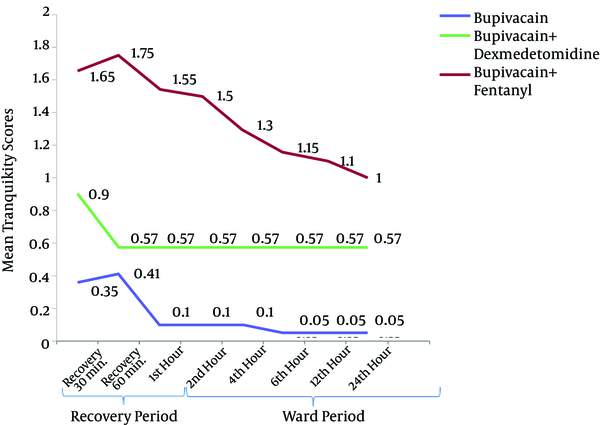
There was a significant difference in the mean sedation scores between different postoperative time points in each group. There was also a significant difference between the groups with regard to the mean sedation scores (P < 0.001) at all time points, with the subsequent post-hoc analysis revealing a significant difference in the mean sedation scores between group B and BD (P < 0.001), group B and BF (0.003 ≤ P ≤ 0.049) and group BD and BF (< 0.001 ≤ P ≤ 0.034). The lowest scores were observed in group B (0.35 ± 0.49) and the highest were observed in group BD (1.65 ± 0.67), whereas the scores in the BF group were intermediate (0.95 ± 0.22) at the 30-min recovery time. The mean sedation scores were not significantly different between group BD and BF only at the postoperative 60-min, 1-h and 24-h time points (Figure 3).
Sedation Scores of the Three Groups (Bupivacaine, Bupivacaine-Dexmedetomidine and Bupivacaine-Fentanyl) After the Operation (Recovery and Ward Period)
4.3. Motor Block
Motor block was not detected in any of the patients after the operation.
4.4. Analgesic Requirement
The incidence of pain was generally low in patients of both groups, except for three patients aged between 12 and 19 months in the bupivacaine group: two 1-year-old children and one 1.5-year-old child. In the 1-year-old children, the first episode of pain occurred at 6 hours after caudal block initiation, and in the 1.5-year-old child, it occurred at 2 hours after caudal block initiation.
4.5. Side Effects
No side effects, such as nausea and vomiting, respiratory depression, pruritis, bradycardia, hypotension, and urinary retention, occurred in any of the study groups.
4.6. Duration of Surgery
The duration of surgery was longer in the BF group, but it was not significantly different from that in the other groups (Table 1).
5. Discussion
The use of caudal analgesics is popular in pediatric operations (12); however, a single shot of one analgesic agent alone may not be sufficient to induce prolonged analgesia, so many multiple modalities have been introduced. The use of caudal catheters in children may affect postoperative mobility or carry the risk of an infection (13). Therefore, it would be beneficial to use a multimodal method with a block solution that has prolonged analgesic effects and does not have any adverse effects on the perioperative vital conditions of the patient.
The aim of our study was to determine the effect of adding other analgesic agents (dexmedetomidine and fentanyl) to a bupivacaine solution for caudal block. The results revealed a significant difference in the efficacy of the drug solution containing additives and bupivacaine alone.
An ideal sedative agent is a one that has the least cardiovascular (e.g., hypotension) and respiratory (e.g., apnea) side effects. In this study, the effects of administering dexmedetomidine and fentanyl as additional sedatives along with bupivacaine were studied. Dexmedetomidine has a dual effect as an α2 adrenergic agonist and an α1 adrenergic antagonist that acts on the arterial vascular system (7). α2 adrenergic stimulation of the brain and spinal cord is associated with sedative, analgesic, anxiolytic and sympatholytic effects. Unlike other sedative agents, the effects of dexmedetomidine can be easily reversed with slight stimulation and no untoward effects on respiratory functions. Further, it has been reported that dexmedetomidine does not cause respiratory depression even at high doses (7). Fentanyl belongs to the same group of opioids as phenyl piperidine; it is a potent short-acting opioid that affects different opioid receptors and may have dose-related side effects such as respiratory depression, pruritis and nausea and vomiting (6).
The analgesic effect of α2 agonists is unique as they act on peripheral tissues as well as the brain, brainstem and spinal cord. The locus coeruleus is a pivotal supraspinal site of action for α2-adrenergic and opioid agents. The effect of these agents on the spinal cord is brought about via activation of the descending medullospinal noradrenergic pathway and presynaptic ganglionic block, which attenuates spinal sympathetic outflow (14).
In a study by Shukla et al. the postoperative analgesic effects of 1 mL/kg 0.25% ropivacaine + 2 µg/kg clonidine were compared to those of 1 mL/kg 0.25% ropivacaine + 1 µg/kg fentanyl: the co-administration of fentanyl was associated with significantly more complications such as respiratory depression, vomiting, and bradycardia (15). Opioids such as fentanyl can migrate through the cerebral spinal fluid to reach chemo-receptors in the brain stem. In particular, in the case of lipophilic opioids such as fentanyl and sufentanil, early respiratory depression may occur during the first 30 minutes after injection and may last for 2 hours (6).
In our study, the overall pain scores were in the lower range. The pain scores were generally lower with dexmedetomidine throughout the study, but the difference compared to the other treatments was only significant in the early phase of the postoperative period. Despite this, the results did not definitively indicate the superiority of dexmedetomidine over fentanyl in preventing postoperative pain.
A study by Gaitini et al. showed that administration of 1 µg/kg fentanyl with 2% lidocaine for caudal epidural block was not beneficial for preventing postoperative pain after circumcision in children (16). However, in our study, the bupivacaine-fentanyl group had significantly better analgesia scores than the bupivacaine only group. This may be related to the difference in the type of local anesthetic used as well as the higher dosage of fentanyl (2 µg/kg) used in our research.
In our study, pain relief was observed earlier in the postoperative course in the BD group than in the BF group: this is probably because caudal application of dexmedetomidine had better analgesic effects than caudal application of fentanyl.
The pharmacokinetics of bupivacaine after induction of caudal anesthesia in children administered 2.5 mg/kg of bupivacaine has been studied by Mazoit et al.: the serum levels were found to be in the range of 0.5 - 1.9 μg/mL, with the peak plasma levels observed 10 - 60 minutes after administration (17). However, the peak levels of caudal dexmedetomidine have not been defined yet. After intravenous administration of dexmedetomidine, the onset time of anesthesia is at 15 minutes after administration, and the peak concentration is attained in approximately an hour under continuous infusion. Further, the terminal half-life of dexmedetomidine is 2 - 3 hours (18). In a study by Koroglu et al. the onset of sedation after intravenous administration of dexmedetomidine was observed at 19 minutes (19).
In a study by She et al. the effective onset times for caudally applied 0.20% levobupivacaine with 2 μg/kg dexmedetomidine were 9.91 min (8.55 - 11.28, 50% confidence interval) and 16.39 min (13.32 - 19.46, 95% confidence interval). The mean duration of analgesia in these children was 19.6 hours (range, 8 - 24 hours) (20). The duration of analgesia reported by them is similar to the values in this study, but we did not measure the onset time because it was during the operation. However, we did measure the peak effect time during the recovery phase, which has not been reported by any other study.
Addition of dexmedetomidine along with levobupivacaine prolongs the duration of analgesia during caudal block in children (20). Epidural dexmedetomidine inhibits the propagation of C-fiber impulses, which affects the hyperpolarization of postsynaptic dorsal neurons. The synergistic effect of dexmedetomidine and local anesthetics produces a blocking effect in the Aδ and C fibers, which lowers the absorption of local anesthetics and hampers the sympathetic system, leaving the cholinergic system uninhibited. Although dexmedetomidine is involved in these complex mechanisms, the concentration of the local anesthetic administered may have an important effect on the pharmacokinetics of the analgesic (20).
The average duration of anesthesia was 58 minutes in the BD group, and the end point of anesthesia in this group coincided with the peak effect of dexmedetomidine. However, the pain scores decreased till the 6th hour of ward stay or 7 hours after the end of the operation. If 20 min are added to account for the time required for anesthesia and block initiation, it means that the analgesic effect of causal dexmedetomidine peaked at 440 minutes in the recovery period.
In agreement with our findings, a number of other studies have shown that administering dexmedetomidine with bupivacaine has better analgesic and sedative effects than administering bupivacaine alone (21-23), and one study has also shown that administering fentanyl with bupivacaine has better analgesic and sedative effects than bupivacaine alone (24).
In one study, 0.25% bupivacaine (1 ml kg-1) with either 2 µg kg-1 dexmedetomidine or clonidine was used in pediatric lower abdominal surgery. Even though dexmedetomidine has eight times (25) more affinity for α2 receptors than clonidine, no difference was observed in the efficiency of postoperative analgesia between these two agonists. However, the analgesic profile was significantly better with these additives than with bupivacaine alone (26).
In our study, the overall analgesia and sedation scores were generally less than 2, with the best sedation scores found in the BD group: the level of sedation decreased significantly in descending order of the BD, BF and B group. In supporting to these findings improved sedation and pain scores with dexmedetomidine have also been shown with intratechal administration of BD too (27).
Motor block was not observed in any of the patients in our study. However, it has been shown that clonidine, which is also an α2 agonist, has the ability to increase the time period and intensity of the motor block when it is intrathecally administered with bupivacaine for cesarean section (28). This is believed to be the result of α2 adrenergic stimulation, which may augment the local anesthetic properties first by enhancing potassium efflux from neuronal A-delta and C-type fibers and then resulting in a depressed action potential; thus, the increase in the vasoconstrictory effect of α2 agonists decreases the absorption of local anesthetic agents from the blocked area (29, 30).
The motor block could be related to the type of agents used. For instance, in one study, administration of 0.1% ropivacaine (1 ml kg-1) and clonidine (2 µg kg-1) did not induce a motor block (31). This may be due to the dilution effect on the local anesthetic agent in the caudal epidural space. In agreement with the results of our study, the study by She et al. also reported that the postoperative motor block was not augmented by the addition of dexmedetomidine (20). Further, in a recent study, intrathecal administration of 0.5% bupivacaine (2.5 mL) + 10 µg dexmedetomidine was associated with significantly more efficient analgesia and motor block than intrathecal administration of 0.5% bupivacaine (5 mL) + 25 µg fentanyl (0.5 mL) (8).
We expected to observe respiratory depression in the fentanyl-bupivacaine group, but respiratory depression was not observed during the postoperative period in any of our study groups. Similarly, it has been reported that the respiratory indices are similar in the BD group and B group (21, 26, 32). In a study by Rathmell et al. it was found that caudal administration of BF did not have respiratory depressive effects (33). Thus, the findings of these studies are in agreement with those of the present study (33).
No hypotension or bradycardia was observed in our patients. In contrast, Al-Zaben et al. reported a 6.67% incidence of bradycardia in their study, which may be related to the dose of the drugs used and/or the type of surgery performed (22). Wu et al. have also reported that using dexmedetomidine as an adjuvant for inducing neuroaxial anesthesia can cause bradycardia without profound hypotension (34). However, in agreement with our present findings, a number of other studies (21, 24) have also reported that postoperative vomiting and nausea were not observed.
Similar to the results of the studies by Umarani et al. and Raval et al. (23, 35), in our study, urinary retention was not observed. However, the Al-Zaben et al. study (22) reported a 3.44% incidence of urinary retention.
For caudal block, administration of 1 to 2 μg/kg of dexmedetomidine with bupivacaine prolongs the duration of analgesia without significant side effects and also reduces the onset time of sensory-motor block, the total dose of analgesics required and the chances of postoperative shivering. Delaying motor regression and need of first rescue analgesic. Prolonging the duration of sensory block and postoperative analgesia (36) as in our results.
5.1. Conclusions
The results of our study show that administration of bupivacaine-dexmedetomidine was more beneficial than administration of bupivacaine-fentanyl or bupivacaine alone with regard to inducing analgesia and sedation in the group of 1- to 5-year-old children examined after elective inguinal hernia repair. No side effects, such as motor block, hypotension, bradycardia, pruritis, urinary retention and nausea and vomiting, were observed in our study groups.
5.2. Limitations
The patients were admitted to different wards in the children’s hospital, which may have caused a bias in their postoperative follow-up and evaluation findings. Moreover, we did not anticipate or record the onset time of analgesia after the application of the caudal block.
5.3. Recommendations
Although a number of studies have investigated the effects of caudal block with bupivacaine and dexmedetomidine, none of these studies ran comparisons with a third group (like the bupivacaine-fentanyl group in this study).
Acknowledgements
References
-
1.
Rahimzadeh P, Safari S, Imani F. Pediatric Chronic Pain Management: Steps Toward a Neglected Area. J Comprehens Pediatr. 2012;4(1):47-8.
-
2.
Giaufre E. Caudal anesthesia in children. Cahiers d'anesthesiologie. 1994;43(3):281-6.
-
3.
Khalil S, Campos C, Farag AM, Vije H, Ritchey M, Chuang A. Caudal block in children: ropivacaine compared with bupivacaine. Anesthesiology. 1999;91(5):1279-84. [PubMed ID: 10551577].
-
4.
Heshmati F, Zeinaly MB, Mohammadzadeh K, Mahoori AR. Intra-articular Bupivacaine versus Bupivacaine plus Dexamethasone for Analgesia after Knee Surgery. Iran Red Crescent Med J. 2005;2006(1):39-43.
-
5.
Kumar P, Rudra A, Pan AK, Acharya A. Caudal additives in pediatrics: a comparison among midazolam, ketamine, and neostigmine coadministered with bupivacaine. Anesth Analg. 2005;101(1):69-73. table of contents. [PubMed ID: 15976208]. https://doi.org/10.1213/01.ANE.0000153862.95153.2E.
-
6.
Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Cohen NH, Young WL. Miller's anesthesia. Elsevier Health Sciences; 2014.
-
7.
Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85(5):1136-42. [PubMed ID: 9356115].
-
8.
Tarbeeh GA, Mohamed AA. Effects of intrathecal bupivacaine–fentanyl versus bupivacaine–dexmedetomidine in diabetic surgical patients. Egypt J Anaesthesia. 2013;29(1):13-8.
-
9.
Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17-24. [PubMed ID: 18487245]. https://doi.org/10.1093/bja/aen103.
-
10.
Brenner L, Kettner SC, Marhofer P, Latzke D, Willschke H, Kimberger O, et al. Caudal anaesthesia under sedation: a prospective analysis of 512 infants and children. Br J Anaesth. 2010;104(6):751-5. [PubMed ID: 20385572]. https://doi.org/10.1093/bja/aeq082.
-
11.
McNamee DA, Parks L, McClelland AM, Scott S, Milligan KR, Ahlen K, et al. Intrathecal ropivacaine for total hip arthroplasty: double-blind comparative study with isobaric 7.5 mg ml(-1) and 10 mg ml(-1) solutions. Br J Anaesth. 2001;87(5):743-7. [PubMed ID: 11878526].
-
12.
Hosseini SA, Poor Sadegh S, Hosseini Valami SM, Javadi A. Effects of suppository acetaminophen, bupivacaine wound infiltration, and caudal block with bupivacaine on postoperative pain in pediatric inguinal herniorrhaphy. Anesth Pain Med. 2012;2012(4, Spring):243-7.
-
13.
Ansermino M, Basu R, Vandebeek C, Montgomery C. Nonopioid additives to local anaesthetics for caudal blockade in children: a systematic review. Paediatr Anaesth. 2003;13(7):561-73. [PubMed ID: 12950855].
-
14.
Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98(1):153-8. table of contents. [PubMed ID: 14693611].
-
15.
Shukla U, Prabhakar T, Malhotra K. Postoperative analgesia in children when using clonidine or fentanyl with ropivacaine given caudally. J Anaesthesiol Clin Pharmacol. 2011;27(2):205-10. [PubMed ID: 21772681]. https://doi.org/10.4103/0970-9185.81842.
-
16.
Gaitini LA, Somri M, Vaida SJ, Yanovski B, Mogilner G, Sabo E, et al. Does the addition of fentanyl to bupivacaine in caudal epidural block have an effect on the plasma level of catecholamines in children? Anesth Analg. 2000;90(5):1029-33. [PubMed ID: 10781448].
-
17.
Mazoit JX, Denson DD, Samii K. Pharmacokinetics of bupivacaine following caudal anesthesia in infants. Anesthesiology. 1988;68(3):387-91. [PubMed ID: 3344993].
-
18.
Valitalo PA, Ahtola-Satila T, Wighton A, Sarapohja T, Pohjanjousi P, Garratt C. Population pharmacokinetics of dexmedetomidine in critically ill patients. Clin Drug Investig. 2013;33(8):579-87. [PubMed ID: 23839483]. https://doi.org/10.1007/s40261-013-0101-1.
-
19.
Koroglu A, Demirbilek S, Teksan H, Sagir O, But AK, Ersoy MO. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary results. Br J Anaesth. 2005;94(6):821-4. [PubMed ID: 15764627]. https://doi.org/10.1093/bja/aei119.
-
20.
She YJ, Xie GT, Tan YH, Kuang XH, Yu GF, Lian GH, et al. A prospective study comparing the onset and analgesic efficacy of different concentrations of levobupivacaine with/without dexmedetomidine in young children undergoing caudal blockade. J Clin Anesth. 2015;27(1):17-22. [PubMed ID: 25468576]. https://doi.org/10.1016/j.jclinane.2014.09.005.
-
21.
El-Rahmawy GF, Hayes SMS. Efficacy of dexmedetomidine addition to bupivacaine on the quality of blind fascia iliaca compartment block in children undergoing femur fracture surgery. Egypt J Anaesthesia. 2013;29(2):137-42.
-
22.
Al-Zaben KR, Qudaisat IY, Abu-Halaweh SA, Al-Ghanem SM, Al-Mustafa MM, Alja'bari AN, et al. Comparison of caudal bupivacaine alone with bupivacaine plus two doses of dexmedetomidine for postoperative analgesia in pediatric patients undergoing infra-umbilical surgery: a randomized controlled double-blinded study. Paediatr Anaesth. 2015;25(9):883-90. [PubMed ID: 26033312]. https://doi.org/10.1111/pan.12686.
-
23.
Umarani V, Manjunath Patil SSN, Kurbet S, Misra S. Efficacy of dexmedetomidine as an adjuvant to bupivacaine for caudal analgesia in paediatric patients undergoing lower abdominal surgeries. J Evolut Med Dent Sci. 2014;3(28):7653-8.
-
24.
Baris S, Karakaya D, Kelsaka E, Guldogus F, Ariturk E, Tur A. Comparison of fentanyl-bupivacaine or midazolam-bupivacaine mixtures with plain bupivacaine for caudal anaesthesia in children. Paediatr Anaesth. 2003;13(2):126-31. [PubMed ID: 12562485].
-
25.
Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7(4):221-6. [PubMed ID: 11571417].
-
26.
El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103(2):268-74. [PubMed ID: 19541679]. https://doi.org/10.1093/bja/aep159.
-
27.
Safari F, Aminnejad R, Mohajerani SA, Farivar F, Mottaghi K, Safdari H. Intrathecal Dexmedetomidine and Fentanyl as Adjuvant to Bupivacaine on Duration of Spinal Block in Addicted Patients. Anesth Pain Med. 2016;6(1). e26714. [PubMed ID: 27110524]. https://doi.org/10.5812/aapm.26714.
-
28.
Braga AA, Frias JA, Braga FS, Poterio GB, Hirata ES, Torres NA. Spinal anesthesia for cesarean section. Use of hyperbaric bupivacaine (10mg) combined with different adjuvants. Rev Bras Anestesiol. 2012;62(6):775-87. [PubMed ID: 23176986]. https://doi.org/10.1016/S0034-7094(12)70178-2.
-
29.
Elia N, Culebras X, Mazza C, Schiffer E, Tramer MR. Clonidine as an adjuvant to intrathecal local anesthetics for surgery: systematic review of randomized trials. Reg Anesth Pain Med. 2008;33(2):159-67. [PubMed ID: 18299097]. https://doi.org/10.1016/j.rapm.2007.10.008.
-
30.
Alves T, Braz JRC, Vianna PTG. α2-agonistas em anestesiologia: aspectos clínicos e farmacológicos. Revista Brasileira de Anestesiologia. 2000:396-404.
-
31.
Ivani G, De Negri P, Conio A, Amati M, Roero S, Giannone S, et al. Ropivacaine-clonidine combination for caudal blockade in children. Acta Anaesthesiol Scand. 2000;44(4):446-9. [PubMed ID: 10757579].
-
32.
Raval DL, Kartik N. A comparative study between dexmedetomidine and clonidine used as adjuncts to bupivacaine for post-operative analgesia by caudal block in paediatric patients. Asian Pac J Health Sci. 2014;1(2):131-6.
-
33.
Rathmell JP, Lair TR, Nauman B. The role of intrathecal drugs in the treatment of acute pain. Anesth Analg. 2005;101(5 Suppl):S30-43. [PubMed ID: 16334491].
-
34.
Wu HH, Wang HT, Jin JJ, Cui GB, Zhou KC, Chen Y, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9(3). e93114. [PubMed ID: 24671181]. https://doi.org/10.1371/journal.pone.0093114.
-
35.
Raval DL, Kartik N. A comparative study between dexmedetomidine and clonidine used as adjuncts to bupivacaine for post-operative analgesia by caudal lock in paediatric patients. Asian Pac J Health Sci. 2014;1(2):131-6.
-
36.
Solanki SL, Goyal VK. Neuraxial dexmedetomidine: wonder drug or simply harmful. Anesth Pain Med. 2015;5(2). e22651. [PubMed ID: 25866711]. https://doi.org/10.5812/aapm.22651.