Abstract
Background:
Pruritus is a troublesome side effect of intrathecal opioids. Midazolam can reinforce GABA-mediated inhibition of the medullary dorsal horn neurons, and thus theoretically has potential to suppress opioid-induced pruritus.Objectives:
This prospective double-blinded randomized trial aimed at comparing the effects of propofol, midazolam, and a combination of the two on the prevention of pruritus induced by intrathecal sufentanil.Methods:
Eighty-four patients undergoing spinal anesthesia with 3 mL hyperbaric bupivacaine 0.5% and 5 μg sufentanil (1 mL) were randomly allocated to one of the three study groups: Group 1, who were administered 20 mg intravenous (IV) propofol bolus, then 50 μg/kg/min IV infusion; Group 2, who were administered 0.03 mg/kg IV midazolam bolus, then 0.02 mg/kg/h IV infusion; and Group 3, who were administered 10 mg IV propofol and 0.015 mg/kg IV midazolam bolus, then 25 μg/kg/min propofol and 0.01 mg/kg/h midazolam IV infusion. The incidence rates and severity of pruritus were assessed intraoperatively and postoperatively for 24 hours.Results:
The Ramsay Sedation Score was highest for the propofol group throughout the duration of the anesthetic process. Overall, 17 patients in the propofol group (60.7%), eight patients in the midazolam group (28.6%), and nine patients in the propofol-midazolam group (32.1%) developed pruritus (P = 0.027). Intraoperative pruritus was observed in seven patients in the propofol group (25%), two patients in the midazolam group (7.1%), and five patients in the midazolam-propofol group (17.9%) (P = 0.196). Postoperative pruritus developed in 12 patients in the propofol group (42.9%), six patients in the midazolam group (21.4%), and four patients in the midazolam-propofol group (14.3%) (P = 0.041). There was no significant difference between the groups with respect to the severity of pruritus (P > 0.05).Conclusions:
This study showed that in comparison with propofol, the administration of 0.03 mg/kg IV midazolam bolus followed by 0.02 mg/kg/h could be more effective in the prevention of intrathecal sufentanil-induced pruritus without increasing sedation and other side effects.Keywords
1. Background
Intrathecal opioids are frequently administered to patients undergoing major general, thoracic, orthopedic, urologic, and gynecological surgeries to provide postoperative analgesia (1). Regarding their synergistic interaction with local anesthetics, the addition of opioids obviates the need for higher doses of an anesthetic. Opioids also prolong the duration of anesthesia, and provide faster sensory block (2). Pruritus is a troublesome side effect of the intrathecal administration of opioids, which is reported in 20% - 100% of patients in the perioperative period (3, 4). Numerous mechanisms have been proposed to explain the exact causes of opium-induced pruritus, including stimulation of an “itch center” in the central nervous system, activation of the medullary dorsal horn and antagonism of the inhibitory transmitters, and activation of serotonin receptors in the medulla (5, 6). Since non-histamine-releasing opioids can also cause pruritus, it seems that this phenomenon is not the result of histamine release (6-8). However, the exact mechanism involved is not yet clear.
It is thought that the spinal trigeminal nucleus acts as an itch center that is rich in serotonin (5-HT3) receptors, which may be activated by opioids and therefore induce pruritus (9). A number of drugs such as propofol, antihistamines, opiate antagonists, and serotonin-receptor antagonists have been used to control this bothersome symptom, but the results have been conflicting (5, 6). The effect of propofol is a result of the inhibition of signal transmission in the dorsal horn neurons of the spinal cord. Midazolam, a short acting benzodiazepine, can also reinforce GABA-mediated inhibition of the medullary dorsal horn neurons, and thus it has theoretical potential to suppress the opioid-induced pruritus caused by μ-opioid receptor stimulation.
2. Objectives
We designed this study to compare the effects of propofol, midazolam, and a combination of the two on the prevention of pruritus induced by intrathecal sufentanil. We hypothesized that midazolam is more effective than propofol in preventing pruritus caused by the intrathecal administration of sufentanil.
3. Methods
This study was conducted at Imam Khomeini University Hospital in Tehran, Iran. After approval by the ethics committee of Tehran University of Medical Sciences and registration on the Iranian registry of clinical trials (http://irct.ir/) with the number IRCT201203059213N1 (Date registered: July 23, 2013), and after obtaining written informed consent from the patients, 84 ASA I-III patients aged 18 to 60 years scheduled for elective major surgery of the lower limbs, urologic surgery, or inguinal hernia surgery were enrolled in this prospective double-blinded randomized trial. We excluded patients with any contraindication of spinal anesthesia (such as lumbar skin infection, coagulopathy, and increased intracranial pressure), opium addiction, non-elective surgery, known allergy to any of the study drugs, severe chronic obstructive pulmonary disease, or pruritic dermatologic or systemic disease. In a pilot study, the incidence of pruritus associated with intrathecal opioid administration was estimated at approximately 88%. We aimed at a 50% reduction in the incidence of pruritus, and hence with a confidence interval of 0.05 with a power of 80%, the sample size was calculated as 25 in each group. To compensate for potential patient exclusion, we initially enrolled 28 patients in each group.
In the operating theater, a 20-gauge intravenous cannula was inserted into each patient. All patients received 500 ml of normal intravenous (IV) saline preoperatively. To induce spinal anesthesia, each patient received an intrathecal injection of 3 ml hyperbaric bupivacaine 0.5% (Marcaine 0.5% Spinal, AstraZeneca, UK) with 5 µg sufentanil (Sufentanil-Hameln 5 µg/mL, Hameln Pharmaceuticals GmbH, Germany) (1mL) over 15 seconds with the use of a 25-gauge needle. Injection was performed through L3-4, L4-5, or L5-S1 interspaces, based on the surgical site, with the patient in a sitting position. Sensory block was assessed through a pinprick test, and the time needed to reach the highest sensory level was recorded. Motor block was evaluated using the Bromage scale 30 minutes after anesthesia induction, and each patient was assigned a score as follows: 0 = no motor block: full flexion of knee and foot; 1 = partial block: unable to raise extended leg, and just able to move knee; 2 = almost complete block: unable to flex knee, and able to move foot only; 3 = complete block: unable to flex ankle joint, and unable to move knee or foot (10).
When the desired sensory and motor block was achieved, patients were allocated to one of the three study groups by using computer-generated random code numbers which had been placed in opaque, sealed envelopes by a person not involved in the study. The propofol group received 20 mg IV propofol (Pofol, Dongkook Pharmaceuticals, Korea) bolus, then 50 µg/kg/min propofol IV infusion. The midazolam group received 0.03 mg/kg IV midazolam (Midamax 5mg/mL, Tehran Chemie Pharmaceuticals, Iran) bolus, then 0.02 mg/kg/h midazolam IV infusion. The propofol-midazolam group received 10 mg IV propofol and 0.015 mg/kg IV midazolam bolus, then 25 µg/kg/min propofol and 0.01 mg/kg/h midazolam IV infusion. Since all of the patients undergoing spinal anesthesia in our center received some sort of IV sedation, we had no control group.
Demographic characteristics including gender, age, weight, height, BMI, and the ASA class were recorded. Patients were monitored continuously during the operations for heart rates, systolic and diastolic blood pressures, respiratory rates, and peripheral capillary oxygen saturation (SpO2) with noninvasive techniques. Systolic blood pressure (SBP) < 90 or a 20% drop in SBP from baseline were regarded as hypotension, and intravenous fluid was administered as needed. A respiratory rate of nine or less or SpO2 < 90% were interpreted as indicators of respiratory depression. Patients’ levels of consciousness were assessed during the operations based on the Ramsay sedation score (11). Pruritus, which was the main variable of our study, was defined as a sensation provoking the urge to scratch (6). Incidence and severity of pruritus were assessed every 30 minutes intraoperatively by a nurse anesthetist, and every three hours postoperatively for 24 hours by two trained nurses who were not informed of the patients’ groups. The mean values were recorded for both time periods. The patients were questioned on pruritus, and its severity was quantified using a 10-point visual analogue scale (VAS, with 0 presenting no pruritus and 10 presenting the severest imaginable pruritus) and a 4-point Verbal Rating Scale (VRS, with 0 representing no pruritus and 4 the severest pruritus).
Certain factors may confound assessment of pruritus by patients. Therefore, we used two scales to increase the precision of the evaluation as recommended by Phan et al. (12). The incidence rates of other side effects including nausea, vomiting, and respiratory depression were also recorded. The need for anti-emetic or analgesic drugs was also recorded as well. Upon a patient’s request for anti-pruritic or anti-emetic treatment, 4 mg ondansetron (Demitron, Tehran Chemie Pharmaceuticals, Iran) was administered intravenously to alleviate pruritus, and metoclopramide (Pladic, Caspian Tamin Pharmaceuticals, Iran) was used to treat vomiting. The study drugs were prepared and administered by a nurse anesthetist not involved in the study. The patients were also not informed about which drug was administered. Allocation, blinding, and prevention of loss up to follow-up were managed by two anesthesiologists.
3.1. Statistical Analysis
Data were analyzed using SPSS version 20 (SPSS Inc., Chicago, IL, USA). The results were expressed as mean ± standard deviation (SD) or frequency (percent) where appropriate. Kruskal-Wallis and chi-squared tests were used to compare the data where appropriate. A P < 0.05 was considered to be statistically significant.
CONSORT Diagram
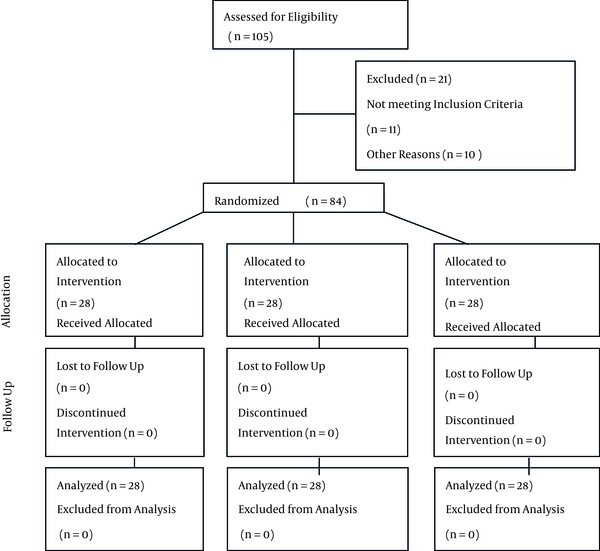
4. Results
A total of 84 patients were finally recruited over the course of 11 months, with 28 patients in each group. Regarding the demographic characteristics, the groups were similar and showed no statistically significant differences. Likewise, operative times and baseline vital signs did not differ among the groups. Characteristics related to anesthesia were compared among the groups. ASA scores, levels of sensory block and the severity of motor block were compared, and the figures showed no statistically significant differences between groups (Tables 1 and 2).
Baseline Characteristics and Intraoperative Data of Patients Receiving Propofol, Midazolam, or Their Combination After Intrathecal Sufentanila
| Propofol (n = 28) | Midazolam (n = 28) | Midazolam-Propofol (n = 28) | P Value | |
|---|---|---|---|---|
| Age, y | 50.9 ± 19 | 47.1 ± 18 | 52.2 ± 18 | 0.557 |
| Gender, (male %) | 96.4 | 85.7 | 100 | 0.321 |
| Height, cm | 169 ± 11 | 168 ± 8 | 170 ± 24 | 0.871 |
| Weight, kg | 71.4 ± 16 | 68.7 ± 8 | 75.1 ± 17 | 0.231 |
| BMI, kg/m2 | 24.3 ± 4 | 24.6 ± 2 | 24.6 ± 4 | 0.922 |
| Mean intraoperative heart rate | 74.1 ± 11 | 71.5 ± 11 | 71.7 ± 16 | 0.677 |
| Mean intraoperative SBP, mmHg | 133 ± 17 | 131 ± 25 | 129 ± 14 | 0.734 |
| Mean intraoperative DBP, mmHg | 83 ± 9 | 81 ± 9 | 83 ± 6 | 0.617 |
| Intraoperative SpO2 (%) | 98.9 ± 2 | 98.6 ± 3 | 99.1 ± 0.6 | 0.079 |
| Duration of anesthesia (drug infusion) (min) | 80 ± 25 | 108 ± 78 | 94 ± 34 | 0.117 |
| ASA score | 0.937 | |||
| ASA I | 20 (71.4) | 19 (67.9) | 22 (78.6) | |
| ASA II | 7 (28.6) | 8 (25) | 5 (17.9) | |
| ASA III | 1 (3.6) | 1 (3.6) | 1 (3.6) |
Level of Sensory and Motor Spinal Block in Patients Undergoing Surgery Following Spinal Anesthesia With Bupivacaine and Sufentanila
| Propofol (n = 28) | Midazolam (n = 28) | Midazolam-Propofol (n = 28) | P Value | |
|---|---|---|---|---|
| Sensory block level | 0.671 | |||
| L3 | 4 (14.3) | 8 (28.6) | 7 (25) | |
| L4 | 16 (57.1) | 15 (53.6) | 16 (57.1) | |
| L5 | 8 (28.6) | 5 (17.9) | 5 (17.9) | |
| Motor block level | 0.272 | |||
| Complete (Bromage 3) | 24 (85.7) | 21 (75) | 22 (78.6) | |
| Almost complete (Bromage 2) | 1 (3.6) | 6 (21.4) | 3 (10.7) | |
| Partial (Bromage1) | 3 (10.7) | 1 (3.6) | 3 (10.7) |
Patients’ levels of consciousness were assessed every 30 minutes from the beginning of the anesthesia and scored according to the Ramsey Sedation Scale. The results showed that throughout this period, the median sedation score of the patients in the propofol group was higher than the corresponding rate of the midazolam-propofol group, and it was also higher than the rate for the midazolam group (P < 0.01) (Figure 2).
Median Ramsay Sedation Scores for Patients Receiving Sedation With Propofol, Midazolam, or Their Combination During Their Procedures (P< 0.01)
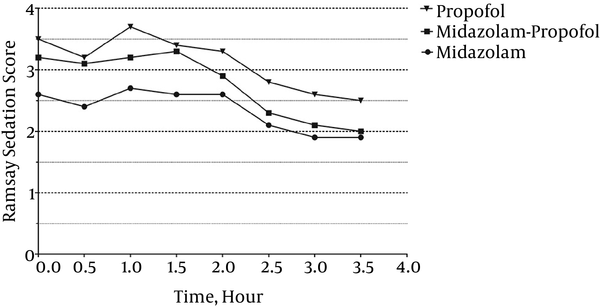
The incidence rates and severity of pruritus were assessed using VAS and VRS. In total, 34 patients developed pruritus, of which 17 were in the propofol group (group member frequency: 60.7%), eight were in the midazolam group (28.6%), and nine were in the midazolam-propofol group (32.1%) (P = 0.027). Intraoperative pruritus was observed in 14 patients, of which seven were in the propofol group (25%), two were in the midazolam group (7.1%), and five were in the midazolam-propofol group (17.9%) (P = 0.196). Twenty-two patients developed postoperative pruritus, of which 12 were in the propofol group (42.9%), six were in the midazolam group (21.4%), and four were in the midazolam-propofol group (14.3%) (P = 0.041). Note that two patients in the propofol group developed intraoperative pruritus, which also persisted postoperatively. The percentage of patients who developed pruritus in each group is depicted in Figure 3. Severity of pruritus was assessed using the VAS and the VRS intraoperatively and postoperatively. Neither showed a significant difference among the groups (P > 0.05) (Table 3). Severe intraoperative pruritus necessitated ondansetron administration in one patient in the propofol group. Ondansetron was administered postoperatively for four patients in the propofol group, three patients in the midazolam group, and two patients in the propofol-midazolam group.
Incidence Rates of Pruritus in Patients Receiving Propofol, Midazolam, or Their Combination After Intrathecal Sufentanil Administration (Intraoperative, Postoperative, Intra- and Post-Operative, and No Pruritus)

| Propofol (n = 28) | Midazolam (n = 28) | Midazolam-Propofol (n = 28) | P Value | |
|---|---|---|---|---|
| Intraoperative | ||||
| VAS | 2.7 | 2 | 2.4 | 0.207 |
| VRS | 1.0 | 1.0 | 1.4 | 0.141 |
| Postoperative | ||||
| VAS | 4.2 | 6.8 | 6.5 | 0.137 |
| VRS | 1.8 | 2.5 | 2.5 | 0.456 |
Mean time to developing pruritus after induction of sensory blockade was 120 ± 71 minutes in the propofol group, 292 ± 42 minutes in the midazolam group, and 108 ± 42 minutes in the propofol-midazolam group, which demonstrated significant differences (P = 0.007).
Along with pruritus, the incidence rates of other opioid-related side effects such as nausea, vomiting, hypotension, bradycardia, arrhythmia, and hypoventilation were recorded, and there were not any significant differences between the three groups with respect to these variables (Table 4).
Incidence Rates of Other Opioid-Related Side Effects in Patients Following Intathecal Sufentanila
| Side Effect | Propofol (n = 28) | Midazolam (n = 28) | Midazolam-Propofol (n = 28) | P Value |
|---|---|---|---|---|
| Nausea | 10 (35.7) | 5 (17.9) | 9 (32.1) | 0.294 |
| Vomiting | 6 (21.4) | 5 (17.9) | 7 (25) | 0.809 |
| Need of anti-emetic | 2 (7.1) | 5 (17.9) | 8 (28.6) | 0.112 |
| Hypotension | 6 (21.4) | 2 (7.1) | 3 (10.7) | 0.257 |
| Bradycardia | 5 (17.9) | 1 (3.6) | 2 (7.1) | 0.166 |
| Arrhythmia | 2 (7.1) | 0 | 0 | 0.325 |
| Respiratory rate < 10 | 1 (3.6) | 0 | 2 (7.1) | 0.770 |
| SpO2 < 95% | 3 (10.7) | 0 | 2 (7.1) | 0.362 |
5. Discussion
This study was designed to compare the effects of propofol and midazolam on the prevention of intrathecal sufentanil-induced pruritus. We recruited 84 eligible patients and allocated them to three groups who were then administered propofol, midazolam, or both. The incidence rates and severity of pruritus were assessed intraoperatively and postoperatively. Levels of sedation and incidence rates of other opioid-related side effects were recorded as well.
We found that the incidence rates of overall pruritus in the propofol, midazolam, and propofol-midazolam groups were 60.7%, 28.5%, and 32.1%, respectively (P = 0.027). Although the incidence rate of intraoperative pruritus was lowest in the midazolam group, this difference was not statistically significant. The incidence rate of postoperative pruritus in the midazolam group was significantly lower than that of the other groups (P = 0.041). Overall, mean time to pruritus was also longest for the midazolam group and shortest for the midazolam-propofol group, with a statistically significant difference (P = 0.007). Based on these findings, we concluded that midazolam may be more effective than propofol in the prevention of opium-induced pruritus. Regarding the fact that the Ramsay Sedation Score was highest for propofol-treated patients and lowest for midazolam-treated patients, it seems that the anti-pruritic effect of midazolam should not be attributed to its sedative effect.
Midazolam is a short-acting benzodiazepine used to induce sedation for various procedures and surgeries. It can reinforce GABA-mediated inhibition of the medullary dorsal horn neurons, thus suppressing opioid-induced pruritus caused by μ-opioid receptor stimulation. Studying the efficacy and safety of fospropofol for moderate sedation during colonoscopy, Cohen et al. (13) observed that after pretreatment of patients with IV fentanyl, midazolam was associated with lower rates of pruritus compared with fospropofol. In addition, a case report showed that in a patient with pancreatic adenocarcinoma undergoing palliative treatment whose pruritus was unresponsive to several anti-pruritic medications, application of midazolam successfully controlled the pruritus without induction of sedation (14). Thomsen et al. (15) studied the effects of sedative drugs on the suppression of spontaneous scratching in hairless rats, and reported the positive effectiveness of midazolam on suppression of this cerebral phenomenon. It has also been shown that midazolam inhibits proinflammatory mediators through the inhibition of inducible nitric oxide synthase and cyclo-oxygenase expression in cultured macrophages (16). Regarding the fact that prostaglandin release may be one of the mechanisms involved in opioid-induced pruritus (6), it may be proposed that, along with the GABAergic pathway, midazolam may reduce pruritus through prostaglandin inhibition.
Propofol is a phenol-derivative widely used to induce sedation in surgical and minor procedures. The anti-pruritic effects of this drug are uncertain, and controversial results have been reported (17-21). Warwick et al. (19) reported that subhypnotic doses of propofol are not effective for the prevention of intrathecal morphine-induced pruritus in women undergoing caesarian sections. However, Horta et al. (8) compared the effects of alizapride, propofol, droperidol, and promethazine with saline on the prevention of spinal-morphine-induced pruritus, and showed that the first three drugs successfully reduced the incidence rates of pruritus after spinal administration of morphine, while promethazine was demonstrated to be ineffective. Propofol inhibits medullary dorsal horn transmission, and through this pathway, it may exert an anti-pruritic effect (5, 9, 17, 21). Furthermore, propofol has been shown to have inhibitory effects on cyclo-oxygenase, and this may also contribute to the anti-pruritic effects reported in some studies (22).
Both midazolam and propofol exert their effects by potentiation of GABAA receptors. They have distinct binding sites different from those of GABA (23). Although propofol has been shown to have a hypnotic potency 1.8 times that of thiopental, it has not been compared with midazolam (24). As mentioned above, midazolam and propofol have both been shown to exert anti-inflammatory effects. For instance, Xia et al. compared the anti-inflammatory effects of propofol and midazolam in children undergoing cardiac surgery and showed that propofol is superior to midazolam for the reduction of inflammation and oxidative stress in children with congenital heart disease (25).
It has been reported that simultaneous use of propofol and midazolam has a synergistic effect on their sedative properties (26, 27), but this effect has not been reported for their anti-pruritic effects. In our study, we observed that a combination of midazolam and propofol is less effective than midazolam alone in the prevention of pruritus, which may be due to the lower dose of midazolam used for the former group.
Among other side effects, nausea/vomiting was the most common. Nausea may occur due to stimulation of the chemoreceptor trigger zone and is usually transient. It is noteworthy that propofol and midazolam have been shown to reduce the incidence rates of postoperative nausea and vomiting (28). To some extent, we benefited from this effect in terms of the need to administer anti-emetics. However, some patients may need anti-emetic therapy. Antipsychotics, metoclopramide, serotonin antagonists, antihistamines, and corticosteroids are available drugs to control nausea (29, 30). Despite the fewer side effects of ondansetron, we used metoclopramide in cases of nausea/vomiting. This was to avoid the antipruritic properties of ondansetron and the masking of possible pruritus in these patients. Use of ondansetron was confined to patients who suffered severe pruritus and requested treatment. Since we administered the drug only after presentation of the symptoms and not prophylactically in all patients, we believe that it did not cause remarkable underestimation of the prevalence of pruritus. However, it may have prevented postoperative pruritus in one patient who received ondansetron intraoperatively. Ultimately, we observed that administration of propofol or midazolam did not change the incidence rates of nausea and vomiting. This finding is consistent with those of other studies (31).
In this study, patients’ hemodynamic variables were monitored intraoperatively, and regarding the cardiovascular effects of opioids, no significant difference was observed between the study groups. Gurbulak et al. (32) also found no association between incidence rates of cardiopulmonary events and the use of propofol or midazolam/meperidine. Respiratory depression is a rare side effect of intrathecal opioids caused by suppression of the respiratory centers in the brainstem (33). In our study, a few patients developed hypoventilation, a respiratory rate < 10, or oxygen saturation < 95%, and these figures did not show significant differences between groups.
This study showed that in comparison with propofol, the administration of 0.03 mg/kg IV midazolam bolus, followed by 0.02 mg/kg/h is more effective in prevention of intrathecal sufentanil-induced pruritus without increasing sedation and other side effects. It can therefore be concluded that in the absence of contraindications for midazolam use, this sedative drug is superior to propofol. Although many studies have been conducted to investigate the anti-pruritic effects of propofol, the effects of midazolam have not been adequately studied.
5.1. Limitations
The most important limitation of this study is the relative subjectivity of assessing pruritus, the study variable. This variable cannot be objectively measured by physicians, and we therefore have to rely on patients’ perceptions of the symptom. To minimize error, we used two scales to quantitate pruritus. To reduce observer bias, we trained nurses about data collection, and the nurses who asked patients about the symptoms were blinded to the study group. It should, however, be noted that the administration of multiple drugs in the study may have caused unwanted, neglected drug interactions that could have influenced the findings.
Acknowledgements
References
-
1.
Gwirtz KH, Young JV, Byers RS, Alley C, Levin K, Walker SG, et al. The safety and efficacy of intrathecal opioid analgesia for acute postoperative pain: seven years' experience with 5969 surgical patients at Indiana University Hospital. Anesth Analg. 1999;88(3):599-604. [PubMed ID: 10072014].
-
2.
Unlugenc H, Gunduz M, Guzel B, Isik G. A comparative study on the effects of intrathecal morphine added to levobupivacaine for spinal anesthesia. J Opioid Manag. 2012;8(2):105-13. [PubMed ID: 22616316].
-
3.
Yeh HM, Chen LK, Lin CJ, Chan WH, Chen YP, Lin CS, et al. Prophylactic intravenous ondansetron reduces the incidence of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg. 2000;91(1):172-5. [PubMed ID: 10866907].
-
4.
Krajnik M, Zylicz Z. Understanding pruritus in systemic disease. J Pain Symptom Manage. 2001;21(2):151-68. [PubMed ID: 11226766].
-
5.
Kumar K, Singh SI. Neuraxial opioid-induced pruritus: An update. J Anaesthesiol Clin Pharmacol. 2013;29(3):303-7. [PubMed ID: 24106351]. https://doi.org/10.4103/0970-9185.117045.
-
6.
Szarvas S, Harmon D, Murphy D. Neuraxial opioid-induced pruritus: a review. J Clin Anesth. 2003;15(3):234-9. [PubMed ID: 12770663].
-
7.
Kyriakides K, Hussain SK, Hobbs GJ. Management of opioid-induced pruritus: a role for 5-HT3 antagonists? Br J Anaesth. 1999;82(3):439-41. [PubMed ID: 10434832].
-
8.
Horta ML, Morejon LC, da Cruz AW, Dos Santos GR, Welling LC, Terhorst L, et al. Study of the prophylactic effect of droperidol, alizapride, propofol and promethazine on spinal morphine-induced pruritus. Br J Anaesth. 2006;96(6):796-800. [PubMed ID: 16597655]. https://doi.org/10.1093/bja/ael072.
-
9.
Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67(16):2323-33. [PubMed ID: 17983254].
-
10.
Graham AC, McClure JH. Quantitative assessment of motor block in labouring women receiving epidural analgesia. Anaesthesia. 2001;56(5):470-6. [PubMed ID: 11350336].
-
11.
Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656-9. [PubMed ID: 4835444].
-
12.
Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502-7. [PubMed ID: 22170091]. https://doi.org/10.2340/00015555-1246.
-
13.
Cohen LB, Cattau E, Goetsch A, Shah A, Weber JR, Rex DK, et al. A randomized, double-blind, phase 3 study of fospropofol disodium for sedation during colonoscopy. J Clin Gastroenterol. 2010;44(5):345-53. [PubMed ID: 19996984]. https://doi.org/10.1097/MCG.0b013e3181c2987e.
-
14.
Prieto LN. The use of midazolam to treat itching in a terminally ill patient with biliary obstruction. J Pain Symptom Manage. 2004;28(6):531-2. [PubMed ID: 15589075]. https://doi.org/10.1016/j.jpainsymman.2004.10.001.
-
15.
Thomsen JS, Benfeldt E, Serup J. Suppression of spontaneous scratching in hairless rats by sedatives but not by antipruritics. Skin Pharmacol Appl Skin Physiol. 2002;15(4):218-24. [PubMed ID: 12218283].
-
16.
Kim SN, Son SC, Lee SM, Kim CS, Yoo DG, Lee SK, et al. Midazolam inhibits proinflammatory mediators in the lipopolysaccharide-activated macrophage. Anesthesiology. 2006;105(1):105-10. [PubMed ID: 16810001].
-
17.
Borgeat A, Wilder-Smith OH, Saiah M, Rifat K. Subhypnotic doses of propofol relieve pruritus induced by epidural and intrathecal morphine. Anesthesiology. 1992;76(4):510-2. [PubMed ID: 1550275].
-
18.
Beilin Y, Bernstein HH, Zucker-Pinchoff B, Zahn J, Zenzen WJ. Subhypnotic doses of propofol do not relieve pruritus induced by intrathecal morphine after cesarean section. Anesth Analg. 1998;86(2):310-3. [PubMed ID: 9459240].
-
19.
Warwick JP, Kearns CF, Scott WE. The effect of subhypnotic doses of propofol on the incidence of pruritus after intrathecal morphine for caesarean section. Anaesthesia. 1997;52(3):270-5. [PubMed ID: 9124670].
-
20.
Charuluxananan S, Kyokong O, Somboonviboon W, Lertmaharit S, Ngamprasertwong P, Nimcharoendee K. Nalbuphine versus propofol for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg. 2001;93(1):162-5. [PubMed ID: 11429358].
-
21.
Saiah M, Borgeat A, Wilder-Smith OH, Rifat K, Suter PM. Epidural-morphine-induced pruritus: propofol versus naloxone. Anesth Analg. 1994;78(6):1110-3. [PubMed ID: 8198266].
-
22.
Inada T, Kubo K, Kambara T, Shingu K. Propofol inhibits cyclo-oxygenase activity in human monocytic THP-1 cells. Can J Anaesth. 2009;56(3):222-9. [PubMed ID: 19247743]. https://doi.org/10.1007/s12630-008-9035-0.
-
23.
Peduto VA, Concas A, Santoro G, Biggio G, Gessa GL. Biochemical and electrophysiologic evidence that propofol enhances GABAergic transmission in the rat brain. Anesthesiology. 1991;75(6):1000-9. [PubMed ID: 1660227].
-
24.
Schüttler J, Schwilden H. Modern anesthetics. Berlin: Springer; 2008.
-
25.
Xia WF, Liu Y, Zhou QS, Tang QZ, Zou HD. Comparison of the effects of propofol and midazolam on inflammation and oxidase stress in children with congenital heart disease undergoing cardiac surgery. Yonsei Med J. 2011;52(2):326-32. [PubMed ID: 21319354]. https://doi.org/10.3349/ymj.2011.52.2.326.
-
26.
Short TG, Chui PT. Propofol and midazolam act synergistically in combination. Br J Anaesth. 1991;67(5):539-45. [PubMed ID: 1751266].
-
27.
McClune S, McKay AC, Wright PM, Patterson CC, Clarke RS. Synergistic interaction between midazolam and propofol. Br J Anaesth. 1992;69(3):240-5. [PubMed ID: 1389840].
-
28.
Rasooli S, Moslemi F, Khaki A. Effect of Sub hypnotic Doses of Propofol and Midazolam for Nausea and Vomiting During Spinal Anesthesia for Cesarean Section. Anesth Pain Med. 2014;4(4). ee19384. [PubMed ID: 25346896]. https://doi.org/10.5812/aapm.19384.
-
29.
Swegle JM, Logemann C. Management of common opioid-induced adverse effects. Am Fam Physician. 2006;74(8):1347-54. [PubMed ID: 17087429].
-
30.
Imani F, Zafarghandi-Motlagh M. Postoperative Nausea and Vomiting in Patients Undergoing Laparoscopy. J Minim Invasive Surg Sci. 2013;2(4).
-
31.
Hatamabadi HR, Arhami Dolatabadi A, Derakhshanfar H, Younesian S, Ghaffari Shad E. Propofol Versus Midazolam for Procedural Sedation of Anterior Shoulder Dislocation in Emergency Department: A Randomized Clinical Trial. Trauma Mon. 2015;20(2). ee13530. [PubMed ID: 26290851]. https://doi.org/10.5812/traumamon.13530.
-
32.
Gurbulak B, Uzman S, Kabul Gurbulak E, Gul YG, Toptas M, Baltali S, et al. Cardiopulmonary safety of propofol versus midazolam/meperidine sedation for colonoscopy: a prospective, randomized, double-blinded study. Iran Red Crescent Med J. 2014;16(11). ee19329. [PubMed ID: 25763217]. https://doi.org/10.5812/ircmj.19329.
-
33.
Carvalho B. Respiratory depression after neuraxial opioids in the obstetric setting. Anesth Analg. 2008;107(3):956-61. [PubMed ID: 18713913]. https://doi.org/10.1213/ane.0b013e318168b443.