Abstract
Keywords
1. Introduction
Low back pain (LBP), with or without leg pain is a common clinical condition, affecting patients of all ages. It represents the 5th cause of health-care visits in the USA, leading to a serious impact on the overall health status and quality of life (1, 2). Epidural steroid injections (ESIs) are commonly used in the management of certain cases of chronic low back and leg pain, which is due to radicular symptoms out of disc herniation or spinal stenosis (1-6). The mechanism of action of epidural steroids is mainly anti-inflammatory, through the reduction of pro-inflammatory mediators (6) in addition to stabilization of neural membranes, suppression of ectopic discharges and reduces nociceptive input (6). Multiple studies have shown the efficacy of epidural injections, however, the debate still continues, especially for the long-term outcome (1). Many studies have been performed including the injection of epidural steroids, alone, or with local anesthetics or even hyaluronidase, with positive results (1, 4-11). However, current evidence suggests that efficacy of epidural steroids varies from strong to moderate, based on different reviews (1, 7-10) depending on the methodology of the study, the region involved (lumbar, thoracic or cervical) and the technique performed (interalaminar, transforaminal or caudal; under fluoroscopy or not). Especially for interlaminar injections, the evidence is strong for short-term relief and moderate for long-term relief (1) whenever the case is disc herniation with radiculitis. Long-term improvement of spinal stenosis symptoms remains low (3).
Electromyography (EMG) is a widely available method of objectively assessing nerve root dysfunction (6, 11-17). The performance of an EMG analysis to identify patients eligible for an epidural steroid injection has been partially studied, with conflicting results (6). Some studies revealed no difference of the outcome after an ESI related to the EMG prior to the injection (6), while others support the contrary (12, 14). Due to the fact that these issues require further investigation on a prospective manner, the aim of this study was to investigate the short- and long-term clinical and electromyographic findings of patients with chronic radicular pain due to disc herniation or spinal stenosis, who underwent interlaminar epidural steroid injections under fluoroscopic guidance.
2. Methods
This was a prospective, open-label study, performed after approval from the institutions’ bioethics and research committee. The study was performed according to the principles of the declaration of Helsinki and written informed consent was obtained by all patients who participated. The study protocol included patients who were presented with chronic low back and leg pain, caused by disc herniation or central spinal stenosis, according to the clinical criteria and MRI findings, which persisted for more than 3 months.
Patients were referred for an interventional therapy after at least a 1-month trial of simple analgesics (paracetamol and/or non-steroidal anti-inflammatory drugs) combined or not with adjuvant drugs (gabapentinoids) in doses selected by the physicians who performed the initial assessment. A full medical history was recorded during the baseline visit. Clinical characteristics of pain were assessed using the brief pain inventory (BPI) and the visual analogue scale (VAS 0-10), while neuropathic elements were identified using the DN4 questionnaire. Mobility and functional status were assessed using the Rolland-Morris questionnaire, while the psychosocial factors contributing to the patient’s pain were assessed using the STAI and DASS tools, respectively.
All patients considered for an ESI underwent a lumbar MRI and a baseline EMG. The exclusion criteria included: all red flags for spinal pain (inflammation, malignancy, spinal fracture, cauda equina), serious coexisting neurological disorders, serious co-morbidities (renal or hepatic failure, severe heart failure, severe respiratory disease), rheumatoid arthritis, ankylosing spondylitis or other rheumatological diseases, prior spinal surgery, prior epidural injections, all contraindications to epidural injections, critical level of spinal stenosis, all contraindications to steroids or NSAIDs administration, age < 18 years old, pregnancy, and patient’s refusal.
After baseline clinical assessment, an interlaminar epidural injection was performed under fluoroscopic guidance. The epidural injection was performed as soon as the EMG and the MRI were performed, not exceeding a 10 day time period. The level of injection was one of the maximum clinical and EMG physical dysfunction, and the epidural space was identified using radiopaque contrast medium. The drugs administered by epidural included triamcinolone 40 mg combined to ropivacaine 0.2%, at a total volume of 6 mL. All epidurals were performed by the same 2 experienced pain physicians, using aseptic conditions, via a 18G Tuohy epidural needle. All patients had an intravenous line in place and were under basic hemodynamic monitoring (blood pressure, heart rate, SpO2, ECG). Patients were kept lying for at least 1 hour after the injection. After full mobility and sensory recovery of the lower extremities, they were discharged with detailed instructions. Additional drugs prescribed after this first visit included only paracetamol. A follow-up of patients was performed 15 days after the ESI, and also after 1, 6, and 12 months. If a patient had a positive result after the first injection (> 50% improvement in VAS score), another epidural was performed, according to physicians’ decision. A maximum of 4 epidural injections per 12 months were performed.
2.1. EMG Examination
All EMGs were performed by the same specialized examiner using standard equipment (Medronic “Keypoint” EMG machine, manufacture date: 2008, Medronic/Dantec A/L), with a preinstalled program for automatic screening and enroll of the Quantitative EMG analysis-MUR (Motor Unit Recruitment) and Interference pattern (IP) (14-23). Every examination was based on a sufficient number of muscles (3 different muscles for every nerve root tested) and each patient’s tolerance in examination was kept in mind, in order to minimize pain due to needle piercing.
Spontaneous activity (SA) was used in order to assess radicular damage (18-21, 24). Any persistent SA (defined as lasting more than 2-3 seconds, recorded on a 100msec-EMG time/tape) was considered abnormal (18, 21, 23, 25-27). Using the needle EMG, the MUP (Motor Unit Potential) was recorded as well, in addition to the morphological features of the MUAPs (Motor Unit Action Potentials), which include amplitude, duration, phases, and turns-errations (14, 21, 26-31). In addition, the interference pattern (IP) and the motor unit recruitment (MUR) were assessed during the process of maximum voluntary contraction (MVC), following the principles of the quantitative electromyography.
Electromyographic assessment was considered positive for radiculitis if it demonstrated axonal findings ≥ 2 muscles, suggestive of specific nerve root involvement. Specific electromyographic findings suggesting radicular damage included: the presence of SA, alterations in motor unit recruitment and alterations in the interference patterns diagram (IP/MUR). The improvement of EMG findings (SA, IP/MUR), after the epidural steroid injections, together with the improvement of pain intensity (measured via the VAS 0-10), were the main outcome parameters. The time points evaluated were at baseline, and after 6 and 12 months of therapy.
2.2. Stastistical Analysis
A total number of 40 patients, 20 with spinal stenosis and 20 with radicular low back pain due to disc herniation, were initially planned, based on previous studies, in order to identify possible differences in outcome (12). The total sample of patients was tested for normal distribution of data using the Shapiro-Wilk test of normality, applying the appropriate parametric and non-parametric statistical tests. The efficacy of ESIs on pain and functional status was tested using Anova repeated measures (in variables that exhibited a P value > 0.05 in Shapiro-Wilk test, suggesting a normal distribution), between baseline (0), 6 and 12 months after therapy. Additionally, in order to evaluate the possible statistical difference between those time points, the paired sample t-test or the non-parametric Wilcoxon tests were applied. The 2 subgroups of patients were tested using the appropriate parametric (Independent t-test) or non-parametric tests (Mann Whitney U-test), in all scales of pain and functional status.
In order to assess the possible prognostic value of EMG findings (motor unit recruitment-MUR/interference PatternIP and SA-spontaneous activity), a multivariate regression analysis was performed using as a dependent variable for the improvement of pain (VAS 0-10, BPI subscales) and functional status (Rolland Morris, RM) at 6 and 12 months. For the identification of independent variables, simple linear regression was initially applied, using all the independent variables that exhibited initially a prognostic value. Each model had a maximum number of 6 independent variables. The R square and F factors were assessed in the proposed models, and quality of findings was tested with residual analysis. P values < 0.05 suggested that the applied model was significant.
Demographic characteristics of patients are presented as a number of patients (n) percentages (%), while data of descriptive statistics are presented as mean ± SD or median ((interquartile range) when applicable. Statistical analysis was performed using the IBM SPSS Statistics v.20 for windows, statistical package, (SPSS Inc.Chicago, IL, USA).
3. Results
A total of 46 consecutive patients were initially enrolled. Due to the unavailability of follow up, 39 patients were finally studied during the whole follow-up period, 10 (25.6%) men and 29 (74.4%) women, aged 41 - 88 years (65.9 ± 12.52), of mean weight 74.74 ± 14.09 kg, and of mean height 163.85 ± 9.22 cm. All patients participating in the study had low back pain with radicular symptoms. Unilateral radicular pain due to disc herniation was diagnosed in 20 patients (51.3%), while central spinal stenosis was diagnosed in 19 (48.7%). Duration of pain and number of ESIs performed are presented in Table 1.
Patients’ Characteristics (n = 39) in the Total Sample of Patients As Well As According to Diagnosis (Radicular Pain Due to Disc Herniation or Spinal Stenosis)
| No. (%) | ||
|---|---|---|
| Radicular pain diagnosis | Disc herniation | 20 (51.30) |
| Spinal stenosis | 19 (48.70) | |
| No of ESIs performed | 1 | 1 (2.60) |
| 2 | 15 (38.50) | |
| 3 | 18 (46.20) | |
| 4 | 5 (12.80) | |
| Duration of pain, y | ≤ 1 | 11 (28.20) |
| 1 - 5 | 12 (30.80) | |
| 5 - 10 | 10 (25.60) | |
| > 10 | 6 (15.40) | |
| Spontaneous activity (SA) in EMG analysis | SA L5 R | 20 (66.67) |
| SA L5 L | 16 (53.33) | |
| SA S1 R | 13 (43.33) | |
| SA S1 L | 16 (53.33) |
3.1. Clinical Outcome
In the total sample of patients (n = 39), results revealed a significant improvement in all clinical scales: VAS, RM, DN4, BPI-severity and BPI-interference, except for DASS depression and anxiety subscales (Table 2). Especially regarding the VAS score, improvement was significant during the first 6 months, and non significant at the time point of 6 - 12 months. Results in the 2 subgroups of patients separately, showed a significant improvement of patients of both groups in all clinical outcome measures except for the STAI-2 and DASS depression and anxiety subscales (Table 2).
Comparison of Outcome Measures (Pain Intensity, Functional Status, Brief Pain Inventory Parameters, Stress and Anxiety Levels) in the Total Sample of Patients and According to Subgroup Analysis (Diagnosis of Radicular Pain Due to Disc Herniation or Spinal Stenosis) Between Baseline, 6 Months and 12 Months After Initial Assessmenta
| Outcome Measure | Baseline | 6 mo | 12 mo | P Value | Test | |
|---|---|---|---|---|---|---|
| Total number of patients (n = 39) | VAS | 6.23 (2.76) | 3.21 (1.99) | 1.74 (2.07) | 0.000* | Friedman |
| RM | 16.13 (4.64) | 10.44 (4.50) | 9.15 (5.14) | 0.000* | Anova | |
| DN4 | 5.28 (1.99) | 3.41 (1.90) | 2.77 (2.17) | 0.000* | Friedman | |
| BPI Pain Severity | 6.30 (2.08) | 3.83 (1.82) | 2.42 (2.09) | 0.000* | Friedman | |
| BPI Pain Interference | 6.05 (2.04) | 3.78 (1.87) | 2.68 (2.44) | 0.000* | Friedman | |
| STAI-Gr-1 | 43.74 (11.59) | 39.95 (11.49) | 0.027* | t-test | ||
| STAI-Gr-2 | 44.54 (11.48) | 42.95 (11.27) | 0.037* | Wilcoxon | ||
| DASS Depression | 9.64 (8.45) | 9.03 (10.12) | 0.096 | Wilcoxon | ||
| DASS Anxiety | 10.41 (7.64) | 9.05 (7.69) | 0,092 | Wilcoxon | ||
| DASS Stress | 16.49 (10.00) | 13.26 (9.47) | 0,010* | Wilcoxon | ||
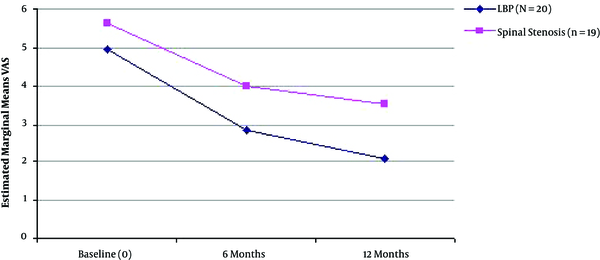
| Disc herniation (n = 20) | VAS | 7.05 (2.33) | 3.15(20.33) | 1.15 (1.78) | 0.000* | Friedman |
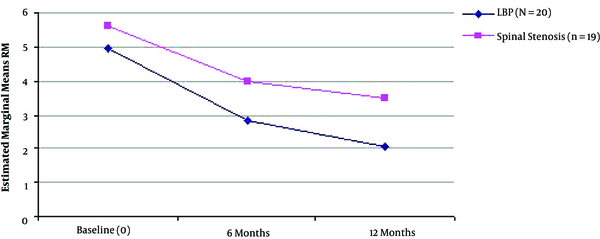
| RM | 16.05 (4.79) | 9.10(4.62) | 7.20(5.22) | 0.000* | Friedman | |
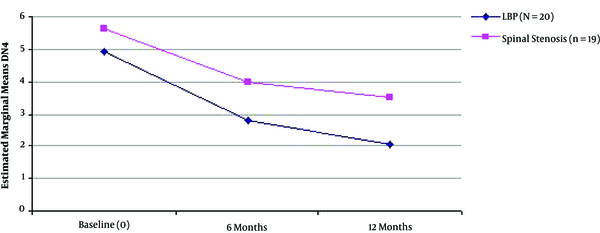
| DN4 | 4.95 (1.57) | 2.85 (1.69) | 2.05 (2.14) | 0.000* | Anova | |
| BPI Pain Severity | 675 (2.14) | 3.72 (1.79) | 1.72 (1.87) | 0.000* | Friedman | |
| BPI Pain Interference | 6.42 (1.94) | 3.40 (1.53) | 1.64 (2.01) | 0.000* | Friedman | |
| STAI-Gr-1 | 46.20 (11.99) | 40.05 (12.04) | 0.007* | t-test | ||
| STAI-Gr-2 | 42.85 (11.42) | 41.15 (11.62) | 0.214 | t-test | ||
| DASS Depression | 8.3 (8.51) | 7.95 (10.51) | 0.409 | Wilcoxon | ||
| DASS Anxiety | 10.4 (8.77) | 9.15 (8.77) | 0.368 | Wilcoxon | ||
| DASS Stress | 15.6 (10.69) | 11.30 (10.37) | 0.015* | Wilcoxon | ||
| Spinal stenosis (n = 19) | VAS | 5.37 (2.97) | 3.26 (1.99) | 2.37 (2.22) | 0.000* | Friedman |
| RM | 16.21 (4.59) | 11.84 (4.02) | 11.21 (4.28) | 0.000* | Friedman | |
| DN4 | 5.63 (2.34) | 4.00 (1.97) | 3.53 (1.98) | 0.000* | Anova | |
| BPI Pain Severity | 5.84 (1.97) | 3.96 (1.88) | 3.17 (2.09) | 0.000* | Anova | |
| BPI Pain Interference | 5.66 (2.12) | 4.17 (2.15) | 3.78 (2.41) | 0.000* | Anova | |
| STAI-Gr-1 | 41.16 (10.88) | 39.84 (11.22) | 0.612 | t-test | ||
| STAI-Gr-2 | 46.32 (11.58) | 44.84 (10.88) | 0.263 | t-test | ||
| DASS Depression | 11.05 (8.37) | 10.16 (9.84) | 0.112 | Wilcoxon | ||
| DASS Anxiety | 10.42 (6.49) | 8.95 (6.60) | 0.072 | t-test | ||
| DASS Stress | 17.42 (9.42) | 15.32 (8.19) | 0.048* | t-test |
3.2. Electromyographic Outcome
Results in the total sample of patients revealed statistically significant improvement in all variables of EMG (IP/MUR & SA) for all nerve roots studied. Improvement was significant mainly for the time point from baseline up to 6 months and from baseline up to 12 months post-treatment, while there were no significant differences observed between 6 to 12 months (Tables 3 and 4). The sub-group analysis of patients revealed that those with radicular pain due to disc herniation showed greater improvement in mean difference of MUR and SA compared to patients with spinal stenosis (mainly for the nerve roots L5-L and S1-L) (Tables 3 and 4).
Electromyographic Changes Including Motor Unit Recruitment During the Maximum Voluntary Contraction and Spontaneous Activity Assessment During the Follow Up Period (12 Months) for the Total Number of Patients (n = 39), As Well As for the Different Subgroups (Patients with Radicular Symptoms Due to Disc Herniation or Spinal Stenosis)a,b
| Months | 0 | 6 | 12 | P Value | Test | |
|---|---|---|---|---|---|---|
| Total number of patients (n = 39) | MUR (IP) L5-R (n = 20) | 69.76 (9.80) | 79.52 (11.61) | 80.48 (11.17) | 0.000* | Friedman |
| MUR(IP) L5-L (n = 16) | 71.33 (9.15) | 78.33 (10.96) | 81 (12.277) | 0.000* | Friedman | |
| MUR(IP) S1-R (n = 13) | 71.54 (11.97) | 80 (12.91) | 81.54 (14.632) | 0.000* | ANOVA | |
| MUR (IP) S1 L (n = 16) | 70.94 (8.98) | 77.5 (6.83) | 80 (9.661) | 0.009* | Friedman | |
| SA L5-R (n = 20) | 3.25 (0.85) | 1.75 (1.16) | 0.80 (1.10) | 0.000* | Friedman | |
| SA L5-L (n = 16) | 3.06 (0.99) | 1.50 (0.97) | 0.75 (1.00) | 0.000* | Friedman | |
| SA S1-R (n = 13) | 3.15 (1.28) | 1.54 (1.33) | 0.85 (1.40) | 0.000* | Friedman | |
| SA S1-L (n = 16) | 3.19 (0.83) | 1.38 (0.96) | 0.69 (0.87) | 0.000* | Friedman | |
| Disc herniation (n=20) | MUR (IP) L5-R (n = 6) | 69.29 (7.32) | 81.43 (8.99) | 84.29 (5.34) | 0.001* | Friedman |
| MUR (IP) L5-L (n = 7) | 70.00 (6.32) | 82.50 (4.18) | 87.50 (4.18) | 0.004* | Friedman | |
| MUR (IP) S1-R (n = 5) | 71.00 (8.94) | 80.00 (7.07) | 84.00 (8.94) | 0.010* | Friedman | |
| MUR (IP) S1-L (n = 6) | 66.67 (5.16) | 80.00 (6.32) | 88.33 (4.08) | 0.004 | Friedman | |
| SA L5-R (n = 6) | 3.33 (0.82) | 2.00 (1.265) | 0.83 (1.33) | 0.005* | Friedman | |
| SA L5-L (n = 7) | 3.29 (0.95) | 1.43 (1.134) | 0.57 (1.13) | 0.002* | Friedman | |
| S1-R (n = 5) | 3.20 (0.84) | 1.00 (1.414) | 0.40 (0.894) | 0.010* | Friedman | |
| SA S1-L (n = 6) | 3.83 (0.408) | 1.33 (1.211) | 0.00 (0.000) | 0.004* | Friedman | |
| Spinal stenosis (n = 19) | MUR L5-R (n = 14) | 70.00 (11.094) | 78.57 (12.924) | 78.57 (12.924) | 0.000* | Friedman |
| MUR L5-L (n = 9) | 72.22 (10.929) | 75.56 (13.333) | 76.67 (14.142) | 0.039* | Friedman | |
| MUR S1-R (n = 8) | 71.88 (14.126) | 80.00 (16.036) | 80.00 (17.728) | 0.003* | Friedman | |
| MUR S1-L (n = 10) | 73.50 (10.014) | 76.00 (6.992) | 75.00 (8.498) | 0.549 | Friedman | |
| SA L5-R (n = 14) | 2.89 (1.054) | 1.56 (0,882) | 0.89 (0.928) | 0,000* | Friedman | |
| SA L5-L (n = 9) | 3.06 (0.99) | 1.50 (0.97) | 0.75 (1.00) | 0.000* | Friedman | |
| SA S1-R (n = 8) | 3.13 (1.55) | 1.88 (1.25) | 1.13 (1.64) | 0.003* | Friedman | |
| SA S1-L (n = 10) | 2.80 (0.79) | 1.40 (0.84) | 1.10 (0.87) | 0.000* | Friedman |
Paired Samples T-Test for Comparison of Changes of Electromyographic Findings in Different Time Points (T2, T3) After Baseline Assessment (T1) in the Two Subgroups of Patients (Radicular Pain Due to Disc Herniation or Spinal Stenosis)a,b
| Paired Wise Test (Wilcoxon) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean change | Disc Herniation (n = 20) | Spinal Stenosis (n = 19) | ||||||
| Mean Difference | P Value | 95% CI for Difference | Mean Difference | P Value | 95% CI for Difference | |||
| MUR L5-R | ||||||||
| T1-T2 | -12.14 | 0,033* | -17.03 | -7.25 | -8.57 | 0,002* | -11.24 | -5.91 |
| T1-T3 | -15.00 | 0,006* | -21.21 | -8.78 | -8.57 | 0.002* | -11.24 | -5.91 |
| T2-T3 | -2.86 | 1.000 | -8.92 | 3.21 | 0,00 | 1.000 | -2.88 | 2.88 |
| MUR L5-L | ||||||||
| T1-T2 | -12.50 | 0.130 | -18.54 | -6.46 | -3.33 | 0.867 | -8.36 | 1.69 |
| T1-T3 | -17.50 | 0.004* | -26.33 | -8.66 | -4.44 | 0.472 | -9.74 | 0.85 |
| T2-T3 | -5.00 | 0.745 | -11.45 | 1.45 | -1.11 | 1.000 | -4.46 | 2.24 |
| MUR S1-R | ||||||||
| T1-T2 | -9.00 | 0.119 | -12.96 | -5.04 | -8.12 | 0.037* | -12.24 | -4.01 |
| T1-T3 | -13.00 | 0.022* | -24.88 | -1.12 | -8.12 | 0,053 | -13.99 | -2.26 |
| T2-T3 | -4.00 | 1.000 | -13.70 | 5.70 | 0,00 | 1,000 | -5.91 | 5.91 |
| MUR S1-L | ||||||||
| T1-T2 | -13.33 | 0.130 | -20.78 | -5.88 | Friedman P value = 0.549 no significant differences between visits. | |||
| T1-T3 | -21.67 | 0.004* | -32.53 | -10.81 | Friedman P value = 0.549 no significant differences between visits. | |||
| T2-T3 | -8.33 | 0.745 | -19.19 | 2.53 | Friedman P value = 0.549 no significant differences between visits. | |||
| SA L5-R | ||||||||
| T1-T2 | 1.33 | 0.250 | 0.15 | 2.51 | 1.57 | 0.003* | 1.02 | 2.13 |
| T1-T3 | 2.50 | 0.007* | 0.98 | 4.01 | 2.43 | 0.000* | 1.63 | 3.23 |
| T2-T3 | 1.17 | 0.582 | -0.25 | 2.58 | 0.86 | 0.896 | -0.05 | 1.76 |
| SA L5-L | ||||||||
| T1-T2 | 1.86 | 0.048* | 0.53 | 3.18 | 1.33 | 0.040* | 0.62 | 2.04 |
| T1-T3 | 2.71 | 0.004* | 0.99 | 4.43 | 2.00 | 0.001* | 0.99 | 3.00 |
| T2-T3 | 0.86 | 1.000 | -0.47 | 2.18 | 0.67 | 0.867 | -0.20 | 1.54 |
| SA S1-R | ||||||||
| T1-T2 | 2.20 | 0.120 | 0.72 | 3.68 | 1.25 | 0.073 | 0.47 | 2,03 |
| T1-T3 | 2.80 | 0.020* | 1.33 | 4.28 | 2.00 | 0.008* | 0.33 | 3,67 |
| T2-T3 | 0.60 | 1.000 | -0.98 | 2.18 | 0.75 | 1.000 | -0.54 | 2.04 |
| SA S1-L | ||||||||
| T1-T2 | 2.50 | 0.130 | 0.51 | 4.49 | 1.40 | 0.005* | 0.92 | 1.88 |
| T1-T3 | 3.83 | 0.004* | 3.24 | 4.42 | 1.70 | 0.001* | 1.07 | 2.33 |
| T2-T3 | 1.33 | 0.745 | -0.41 | 3.08 | 0.30 | 1.000 | -0.33 | 0.93 |
The simple regression analysis revealed that the initial diagnosis, which was disc herniation or spinal stenosis, showed a significant prognostic value, in short-term (VAS P value = 0.042, BPI-interference. P value = 0.007) and long-term clinical improvement of pain (0 - 12) (VAS P value = 0.009 BPI-severity. P value = 0.008, BPI-interference. P value = 0.001). Initial pain scores (measured using the VAS and BPI) also seemed to be of highly prognostic value for all dependent variables. The degree of variability between the dependent variables for the final model ranged between 33.4% - 71.2% (R Sq. = 0.344 and R Sq = 0.712, respectively). Specifically, for each nerve root studied, it was revealed that the independent variable “MUR” had a significant prognostic value for active radicular damage in S1-R root for improvement of pain (BPI-severity: R2 = 0.313, P = 0.047) and functional status (RM: R2 = 0.335, P = 0.038), while for S1-L root only in improvement of pain (VAS: R2 = 0.287, P = 0.032, BPI-severity: R2 = 0.262, P = 0.043, BPI-interference: R2 = 0.262, P = 0.043). The independent variable “SA” also showed a significant prognostic value for patients with S1-L root in improvement of pain (VAS: R2 = 0.277, P = 0.036, BPIseverity: R2 = 0.261, P = 0.043) and functional status (RM: R2 = 0.286, P = 0.033).
Multiple regression analysis with “MUR” and “SA” were set as the dependent variables per nerve root studied and all other factors set as the independent variables, revealed that improvement of functional status (assessed by RM) was significantly correlated with the improvement of MUR in the total sample of patients (for S1-R, R2 = 0.335, P = 0.038 and S1-L, R2 = 0.437, P = 0.005). Similarly, improvement of functional status (measured by RM) was significantly correlated with greater improvement of findings of SA in S1-L root evaluation (R2 = 0.286, P = 0.033) (Table 5 and Figures 1 - 3).
Μultiple Regression Analysis for electromyographic Findings (Including Motor Unit Recruitment and Spontaneous Activity) Between Baseline (T1) and 12 Months After Initial Assessment (T3) for Each Nerve Root Evaluated
| Dependent Variable | Predictive Variables (P Values for Coefficients in Model) | Model Fit (P Value) |
|---|---|---|
| MUR L5-R total change | ||
| Τ1-Τ3 | Improvement in VAS (0.831), RM (0.736), DN4 (0.418) BPIsev (0.724), BRIint. (0.936) | Rsq = 0.091 (0.915) |
| MUR L5-L total change | ||
| T1-T3 | Improvement in VAS (0.476), RM (0.510), DN4 (0.050)* BPIsev. (0.911), BRIint. (0,057) | Rsq = 0.540 (0.118) |
| MUR S1-R total change | ||
| T1-T3 | Improvement in VAS (0.408), RM (0.038)*, DN4 (0.889) BPIsev. (0,337), BRIint. (0.471) | Rsq = 0.335 (0.038)* |
| MUR S1-L total change | ||
| T1-T3 | Improvement in VAS (0.570), RM (0.862), DN4 (0.882) BPIsev (0.854), BRIint. (0.005)* | Rsq = 0.437 (0.005)* |
| SA L5-R total change | ||
| T1-T3 | Improvement in VAS (0.755), RM (0.898), DN4 (0.107), BRIsev (0.523), BPIint. (0.090) | Rsq = 0.369 (0.214) |
| SA L5-L total change | ||
| T1-T3 | Improvement in VAS (0.398), RM (0.948), DN4 (0.470), BRIsev (0.302), BPIint (0.451) | Rsq = 0.191 (0.789) |
| SA S1-R total change | ||
| T1-T3 | Improvement in (0.396), RM (0.858), DN4 (0.504), BPIsev (0.454), BPIint (0.615) | Rsq = 0.330 (0.647) |
| SA S1-L total change | ||
| T1-T3 | Improvement in VAS (0.769), RM (0.033)*, DN4 (0.487), BPIsev (0.702), BPIint (0.691) | Rsq = 0.286 (0.033)* |
Mean Visual Analogue Scale Score (0 - 10) Improvement During the Three Time Points of Assessment in the Two Different Groups of Patients (LBP with Radicular Symptoms, n = 20 and Spinal Stenosis, n = 19).
Mean Rolland Morris Score Improvement During the Three Time Points of Assessment in the Two Different Groups of Patients (LBP with Radicular Symptoms, n = 20 and Spinal Stenosis, n = 19).
Mean DN4 Score Improvement During the Three Time Points of Assessment in the Two Different Groups of Patients (LBP with Radicular Symptoms, n = 20 and Spinal Stenosis, n = 19).
4. Discussion
Low back pain with leg pain represents a very common clinical condition and a significant contributor to chronic pain and disability (1-3). Epidural steroid injections are commonly used in order to manage this condition, although their therapeutic efficacy is still under consideration, especially regarding long-term relief (1-3). The current evidence for interlaminar epidural injections, according to current literature, is strong for short-term relief and moderate for long-term relief, based mostly on clinical signs (1-10). However, studies analyzing the actual electromyographic alterations after ESIs, are still limited.
Results of the current study revealed that epidural steroid injections were effective in reducing the intensity of pain, especially during the first 6 months after treatment, for the majority of patients. The clinical improvement was supported by relevant EMG findings, expressed as motor unit recruitment (MUR) and IP Interference Pattern during the maximum voluntary contraction process, and as for spontaneous activity (SA) improvement during the follow up period. Patients with low back pain due to disc herniation showed greater improvement in EMG variables compared to patients with spinal stenosis, mainly for the L5-L and S1-L nerve roots studied.
There is evidence in literature that in a large percentage of patients with low back pain (up to 57%), many may have an abnormal EMG combined to a normal MRI (20). Therefore, it is still under consideration whether or not an EMG examination prior to an epidural injection may help identify patients who would benefit from the technique. Fish et al. (12) retrospectively studied the predictability and efficacy of epidural injections through the trasforaminal root and revealed that 18 out of 39 patients with radiculopathy proven by EMG prior therapy, the improvement of Oswestry disability index was significantly better after an ESI, compared to patients without EMG findings. Similarly, in the study by Tong et al. (14) the EMG evidence of radiculopathy was related to a better outcome after an ESI, while Chouteau et al. (15) also showed a predictive relationship between EMG findings and long-term outcome after ESIs, but not for short-term. In addition, using the needle electromyography, Annaswamy et al. (32) showed a predictive value as for the long-term improvement of pain but not of the psychosocial parameters.
On the other hand, other studies question the role of the EMG, since the clinical findings are still the most important issue of patients’ outcome. Marchetti et al. (6) studied in a retrospective manner the improvement of pain after epidural steroid injections and if this improvement was correlated with EMG findings. A total of 89 patients were included in this study, all with radiculopathy, and were tested with an EMG 6 months after epidural steroid injections. No significant differences were revealed in patients exhibiting a positive response after the ESI (> 50% pain relief) for EMG findings, since all groups had an improvement in pain scores post-treatment.
Our study, which is one of the few prospective ones on this issue, revealed that EMG findings were improved after ESIs and that EMG could partially help predict their efficacy. The improvement of MUR was significantly correlated with a positive outcome of the pain and functional status of patients. Additionally, patients with disc herniation had a significantly better clinical and electromyographic outcome compared to patients with spinal stenosis. Nevertheless, severe spinal stenosis is a contraindication for epidural steroid injections, and the latest NICE (10) guidelines provide a negative recommendation for ESIs in patients with neurogenic claudication. In our study, these patients were excluded.
In this study, the same examiner and equipment were used in all examinations in order to bypass interpretation differences (21, 31, 33, 34). A main limitation was the absence of randomization of patients', due to the fact that all patients eligible were scheduled for the same treatment; therefore, there was no control group. Additionally, probably a larger number of patients would show more clearly the effect of ESIs on EMG findings; however, the current sample size was based on previous studies on the subject. The inclusion of patients with both spinal stenosis and disc herniation as the cause of radicular pain makes the results more challenging to interpret, given the fact that a most favorable natural history is supposed to occur for unilateral radicular pain due to disc herniation and less so for spinal stenosis. Finally, heterogeneity related to a wide range of duration of pain at presentation is also a limitation.
To conclude, this study revealed that clinical and EMG findings, as assessed by Motor Unit Recruitment and indications of Spontaneous Activity, were improved after epidural steroid injections. Patients with radicular low back pain due to disc herniation exhibited a better outcome compared to patients with spinal stenosis, especially during the first 6 months post-treatment. Future studies may identify the possible differences on outcome, by comparing EMG findings with a control group, and also of different epidural injection techniques, such as interlaminar, transforaminal, parasagittal, or caudal approach. The selection of patients for an epidural steroid injection may be improved in the future, guiding a better management pathway and preventing possibly unnecessary interventions.
Acknowledgements
References
-
1.
Abdi S, Datta S, Trescot AM, Schultz DM, Adlaka R, Atluri SL, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007;10(1):185-212. [PubMed ID: 17256030].
-
2.
Chou R, Qaseem A, Snow V, Casey D, Cross JJ, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-91. [PubMed ID: 17909209].
-
3.
Parr AT, Diwan S, Abdi S. Lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain: a systematic review. Pain Physician. 2009;12(1):163-88. [PubMed ID: 19165302].
-
4.
Imani F, Rahimzadeh P. Interventional pain management according to evidence-based medicine. Anesth Pain Med. 2012;1(4):235-6. [PubMed ID: 24904805]. https://doi.org/10.5812/aapm.4514.
-
5.
Patel VB, Wasserman R, Imani F. Interventional therapies for chronic low back pain, a focused review, (efficacy and outcomes). Anesth Pain Med. 2015;5(4):29716. [PubMed ID: 26484298]. https://doi.org/10.5812/aapm.29716.
-
6.
Marchetti J, Verma-Kurvari S, Patel N, Ohnmeiss DD. Are electrodiagnostic study findings related to a patient's response to epidural steroid injection? PM R. 2010;2(11):1016-20. [PubMed ID: 21093837]. https://doi.org/10.1016/j.pmrj.2010.07.002.
-
7.
Manchikanti L, Boswell MV, Singh V, Derby R, Fellows B, Falco FJ, et al. Comprehensive review of neurophysiologic basis and diagnostic interventions in managing chronic spinal pain. Pain Physician. 2009;12(4):71-120. [PubMed ID: 19668292].
-
8.
Manchikanti L, Cash KA, McManus CD, Damron KS, Pampati V, Falco FJ. Lumbar interlaminar epidural injections in central spinal stenosis: preliminary results of a randomized, double-blind, active control trial. Pain Physician. 2012;15(1):51-63. [PubMed ID: 22270738].
-
9.
Conn A, Buenaventura RM, Datta S, Abdi S, Diwan S. Systematic review of caudal epidural injections in the management of chronic low back pain. Pain Physician. 2009;12(1):109-35. [PubMed ID: 19165299].
-
10.
NICE guideline (NG59). Low back pain and sciatica in over 18s, Assessment and management. invasive treatments. methods, evidence and recommendations. London: NICE; 2016, [cited August 15]. Available from: https://www.nice.org.uk/guidance/ng59.
-
11.
Rahimzadeh P, Sharma V, Imani F, Faiz HR, Ghodraty MR, Nikzad-Jamnani AR, et al. Adjuvant hyaluronidase to epidural steroid improves the quality of analgesia in failed back surgery syndrome: a prospective randomized clinical trial. Pain Physician. 2014;17(1):75-82. [PubMed ID: 24452659].
-
12.
Fish DE, Shirazi EP, Pham Q. The use of electromyography to predict functional outcome following transforaminal epidural spinal injections for lumbar radiculopathy. J Pain. 2008;9(1):64-70. [PubMed ID: 17974488]. https://doi.org/10.1016/j.jpain.2007.08.011.
-
13.
Bierner SM, Annaswamy TM, Chouteau WL. Predictive value of electromyography for response to epidural steroid injection in patients with lumbar radiculopathy. Muscle Nerve. 2009;40:710.
-
14.
Tong HC, Williams JC, Haig AJ, Geisser ME, Chiodo A. Predicting outcomes of transforaminal epidural injections for sciatica. Spine J. 2003;3(6):430-4. [PubMed ID: 14609686].
-
15.
Chouteau WL, Bierner SM, Annaswamy TM. Predictive value of electromyography of response to lumbar epidural steroid injections in patients with lumbar radiculopathy. Am J Phys Med Rehab. 2009;88:6. https://doi.org/10.1016/j.clinph.2008.10.143.
-
16.
Tonzola RF, Ackil AA, Shahani BT, Young RR. Usefulness of electrophysiological studies in the diagnosis of lumbosacral root disease. Ann Neurol. 1981;9(3):305-8. [PubMed ID: 6261675]. https://doi.org/10.1002/ana.410090317.
-
17.
Fisher MA. Electrophysiology of radiculopathies. Clin Neurophysiol. 2002;113(3):317-35. [PubMed ID: 11897532].
-
18.
Delisa JA. Electrodiagnostic evaluation of the peripheral nervous system, (radiculopathy and polyradiculopathy). In: Frontera WR, Delisa JA, editors. . 4th ed. Philadelphia, United States: Lippincott Williams and Wilkins; 2010. 93 p.
-
19.
Levin KH. Electrodiagnostic approach to the patient with suspected radiculopathy. Neurol Clin. 2002;20(2):397-421. [PubMed ID: 12152441].
-
20.
Nardin RA, Patel MR, Gudas TF, Rutkove SB, Raynor EM. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22(2):151-5. [PubMed ID: 10024127].
-
21.
Kimura J. Electrodiagnosis in diseases of nerve and muscle. Philadelphia PA: FA Davis Co; 1989.
-
22.
Aminoff MJ, Goodin DS, Parry GJ, Barbaro NM, Weinstein PR, Rosenblum ML. Electrophysiologic evaluation of lumbosacral radiculopathies: electromyography, late responses, and somatosensory evoked potentials. Neurology. 1985;35(10):1514-8. [PubMed ID: 2993952].
-
23.
Elisen A. Electrodiagnosis of radiculopathies. Neuro Clin. 1985;3(3):494-510. [PubMed ID: 2995782].
-
24.
Adel T, Stashuk D. Clinical quantitative electromyography. University of Waterloo, Canada: Department of System Design Engineering; 2014.
-
25.
Wilbourn AJ, Aminoff MJ. AAEE minimonograph #32: the electrophysiologic examination in patients with radiculopathies. Muscle Nerve. 1988;11(11):1099-114. [PubMed ID: 3067085]. https://doi.org/10.1002/mus.880111102.
-
26.
Henneman E, Somjen G, Carpenter DO. Functional Significance of Cell Size in Spinal Motoneurons. J Neurophysiol. 1965;28:560-80. [PubMed ID: 14328454]. https://doi.org/10.1152/jn.1965.28.3.560.
-
27.
Daube JR. AAEM minimonograph #11: Needle examination in clinical electromyography. Muscle Nerve. 1991;14(8):685-700. [PubMed ID: 1890993]. https://doi.org/10.1002/mus.880140802.
-
28.
Pino LJ, Stashuk DW, Boe SG, Doherty TJ. Motor unit potential characterization using "pattern discovery". Med Eng Phys. 2008;30(5):563-73. [PubMed ID: 17697793]. https://doi.org/10.1016/j.medengphy.2007.06.005.
-
29.
Hodson-Tole EF, Wakeling JM. Motor unit recruitment for dynamic tasks: current understanding and future directions. J Comp Physiol B. 2009;179(1):57-66. [PubMed ID: 18597095]. https://doi.org/10.1007/s00360-008-0289-1.
-
30.
Roeleveld K, Stegeman DF, Falck B, Stalberg EV. Motor unit size estimation: confrontation of surface EMG with macro EMG. Electroencephalogr Clin Neurophysiol. 1997;105(3):181-8. [PubMed ID: 9216486].
-
31.
Kraft GH. Fibrillation potential amplitude and muscle atrophy following peripheral nerve injury. Muscle Nerve. 1990;13(9):814-21. [PubMed ID: 2233867]. https://doi.org/10.1002/mus.880130907.
-
32.
Annaswamy TM, Bierner SM, Chouteau W, Elliott AC. Needle electromyography predicts outcome after lumbar epidural steroid injection. Muscle Nerve. 2012;45(3):346-55. [PubMed ID: 22334168]. https://doi.org/10.1002/mus.22320.
-
33.
DeLuca CJ. Control properties of motor units. In: Basmajian JV, De Luca CJ, editors. . Baltimore: William and Wilkins; 1983. p. 125-67.
-
34.
Stashuk DW. Decomposition and quantitative analysis of clinical electromyographic signals. Med Eng Phys. 1999;21(6-7):389-404. https://doi.org/10.1016/s1350-4533(99)00064-8.