Abstract
Background:
Open prostatectomy is still accompanied by some postoperative bleeding. Prescribing fibrinogen to promote clot formation in patients with bleeding is of critical importance. This research studied the effects of local injection of fibrinogen on level of postoperative bleeding in open prostatectomy.Methods:
Overall, 44 patients were randomly entered in a study on open prostatectomy. Patients in the intervention group received local injections of 500 mg fibrinogen (20 mL) dissolved in distilled water, and the control group patients only received 20 mL of normal saline, where the injections were given by the surgeon at the prostatectomy operation site. All patients were tested for hemoglobin, hematocrit, PT, PTT, INR, and fibrinogen level. Also, the amount of blood loss and requirement for blood products were recorded.Results:
The study groups showed no difference regarding baseline variables. One patient in the fibrinogen group (1.66%) and four patients in the control group (6.66%) received blood products (P < 0.05), and the blood drainage tube at 24 hours after operation was 36.50) 18.70 (mL in the fibrinogen group and 151.36) 120.58 (mL in the control group (P = 0.005). There were no differences in hemoglobin, hematocrit, PT, PTT, INR, and serum fibrinogen level between the groups at any time.Conclusions:
The current study demonstrated that using fibrinogen in patients with high bleeding risk may effectively reduce the amount of bleeding and its subsequent blood transfusion requirement, after open prostatectomy surgery.Keywords
1. Background
Open prostatectomy is one of the prevailing forms of surgery used for treatment of BPH and/or prostate enlargement due to other reasons. Despite extensive experience, this surgical method is still accompanied by a number of risks, including intraoperative and postoperative bleeding (1, 2). Therefore, to reduce or compensate for intraoperative or postoperative bleeding, various methods have been used in patients considered as candidates for prostatectomy (3), including induced hemodilution before surgery (4), erythropoietin injection before surgery (5), prescribing anti-fibrinolytics, such as tranexamic acid (6), and administration of blood products, such as packed red blood cells, fresh frozen plasma, cryoprecipitate, and fibrinogen (7).
Fibrinogen is a glycoprotein produced in the liver, and it is a substrate for thrombin, plasmin, and active factor XIII, with a half-life of three to five days (8). Following tissue damage, fibrinogen is broken down by thrombin, and soluble fibrin monomers are formed, resulting in blood clotting. Thus, fibrinogen is a critical component in the creation, reinforcement, and strengthening of blood clots. Hypofibrinogenemia occurs because of conditions, such as bleeding, hemo-dilution caused by fluid replacement, consumption due to blood clotting, and/or disseminated intravascular coagulation (DIC) (9, 10). Therefore, prescribing fibrinogen to return blood concentration of the fibrinogen to its usual state and to normalize clot formation in patients with bleeding is of critical importance (11-13). Normal levels of fibrinogen in blood were different in various references; however, 2 to 4.5 g/L or 200 to 450 mg/dL is considered normal, on average (14-17). Fibrinogen is used in various forms to control bleeding, including intravenous infusion, through which the drug would be distributed all over the body. In this way, a sufficient amount of drug may not be available to the damaged tissue, thus large amounts of the product would be needed for adequate control of bleeding. Also, it could be used in hemostasis patches, along with collagen and thrombin. This method is accompanied by complications, such as immune responses and kidney problems (18, 19). Therefore, the need for a new way of administering fibrinogen with less drug requirement and complications and more effectiveness has been strongly felt. Considering certain conditions of open prostatectomy and importance of bleeding in these patients, as well as the useful effects of local injection of fibrinogen, which is not prescribed for prostatectomy patients in Iran, the current researchers decided to study the effects of local injection of fibrinogen on the level of postoperative bleeding in open prostatectomy.
2. Methods
After receiving authorization from the ethics committee of Ahvaz Jundishapur University of Medical Sciences, 44 patients (ASA I-II), who were candidates for open prostatectomy, were randomly entered in the study. All patients provided a written consent, after being informed of the procedure. The trial was registered with http://irct.ir (identifier: IRCT20170421033564N1).
A randomization list was generated with computer support. Randomization was determined before surgery, through a sealed opaque envelope system (‘‘Yes’’: Fibrinogen, ‘‘No’’: control group that were prepared by an anesthesiologist). The surgeon and assessor were not aware of the patient grouping. Envelopes were opened at the end of surgery. In the control group, 20 mL of normal saline was injected by the surgeon at the prostatectomy site. In the fibrinogen group, the solution dissolved in 20 mL of distilled water. Randomization envelopes were accounted for at the end of the study. Patients, assessors, and surgeon were blinded to the randomization. During the operation, sterile syringes of both groups that were labeled with number one and two were prepared by an anesthesiologist and were provided to the surgeon. The surgeon and assessor were not aware of the patient grouping. According to Payani et al.’s (20) study and considering α = 0.05, β = 0.2, and mean values s1 = 176.2, s2 = 113.9 and μ1 - μ2 = 150 and the formula:
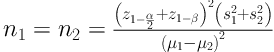
Sample size was calculated as n = 22 for each group.
Criteria for entrance in the study was male gender in the age range of 45 to 70 years old with prostate volume between 50 and 100 mL, and severe urinary symptoms, as well as being a candidate for open prostatectomy. Criteria for exclusion from the study were as follows: any evident coagulation complication; use of anticoagulants; chronic kidney disease with creatinine level higher than 3 mg/dl; record of finasteride use; and refusal of permission for spinal anesthesia. Patients were randomly allocated to two groups: fibrinogen recipients and placebo recipients. They were grouped in terms of size of prostate, age deciles, surgeon, and cardiovascular risk.
Before spinal anesthesia, non-invasive monitors (pulse oximetry, blood pressure, and ECG) were connected, and Ringer’s lactate solution was infused at 5 mL/kg.
Spinal anesthesia was done in the sitting position, at interspaces L4 - L5 and/or L3 - L4, with a 25G Quincke spinal needle, and 12.5 mg Hyperbaric Bupivacaine (HB) was injected. Patients’ blood pressure was measured for the first ten minutes, at three-minute intervals. It was then measured and recorded at five-minute intervals. In case of systolic blood pressure drop to less than 90 mmHg and/or 25% reduction in comparison to the initial blood pressure, 5 mg of intravenous ephedrine was injected. The sensory level of patients was evaluated by the pin-prick method, and the start of surgery was authorized at the T9 level.
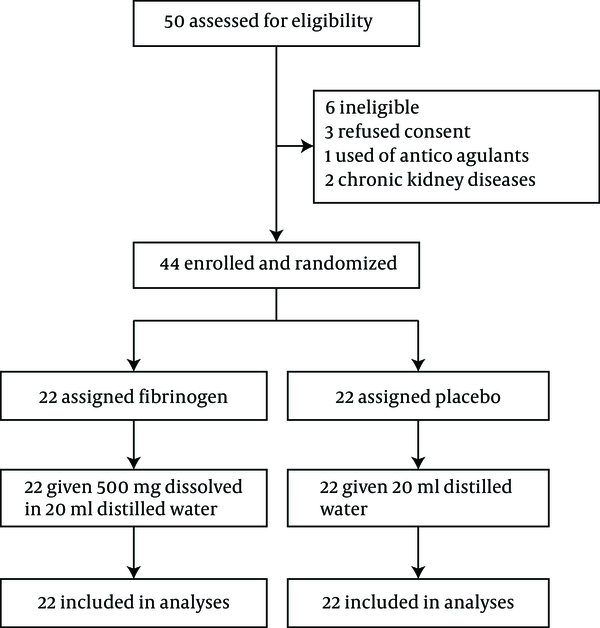
At the end of surgery, the intervention group received 500 mg of fibrinogen: (Haemocomplettan®, CSL Behring GmbH, Marburg, Germany), dissolved in 20 mL of distilled water, and in the control group, the solution was dissolved in 20 mL of normal saline (B Braun , 0.9%, Melsungen, Germany), injected by the surgeon at the prostatectomy site (20). A small closed-suction drain was placed in the pelvic area to prevent hematoma and urinoma formation. A urethral catheter was fixed. Continued bladder irrigation with normal saline solution was initiated to prevent clot formation (21). In the recovery room, the outputs from drain and urethral catheter were monitored. Before surgery, all patients were tested for hemoglobin, hematocrit, PT, PTT, INR, and serum fibrinogen level. Hemoglobin and hematocrit levels were retested at two and twelve hours after surgery, while PT, PTT, and INR were determined in the ward. The output fluid from the drain and urethral catheter was monitored until it was clear and free of blood. The volume of irrigated normal saline solution and the amount of transfused blood products (packed red blood cells, fresh frozen plasma, and platelets) were recorded (Figure 1).
Trial profile
3. Results
All data were analyzed with the t-test and chi-square test. There were no statistically significant differences between the two groups regarding demographic data and underlying diseases (P > 0.05) (Table 1).
| Variable | Control (N = 22) | Fibrinogen (N = 22) | P Value |
|---|---|---|---|
| Age, y | 58.62 (12.31) | 65.09 (6.25) | 0.71 |
| Weight, kg | 70.40 (12.41) | 75.41 (11.84) | 0.32 |
| Height, cm | 165.43 (10.77) | 170.11 (9.03) | 0.48 |
| Diabetic | 4 | 4 | 0.96 |
| Hypertension | 3 | 5 | 0.57 |
| Ischemic Heart Disease | 1 | 1 | 0.1 |
Intraoperative and postoperative bleeding status in both groups is shown in Table 2. According to the results, there was no significant difference between the groups in the amount of blood transfused during surgery (P > 0.05). In the postoperative period, there were significant differences between the groups, both in terms of the number of recipient patients and number of blood units received. Those patients, who received fibrinogen, needed less blood (P < 0.05).
| Variable | Fibrinogen (N = 22) | Control (N = 22) | P Value |
|---|---|---|---|
| Pack Cell (during surgery) | |||
| Number of patient | 18 | 19 | 0.84 |
| Unit | 21 | 28 | 0.67 |
| Pack Cell (post surgery) | |||
| Number of patient | 1 | 4 | 0.038 |
| Unit | 1 | 5 | 0.024 |
| Bleeding (during surgery) | 660.90 (401.36) | 731.36 (379.06) | 0.56 |
| Bleeding (post surgery) | 36.50 (18.70) | 151.36 (120.58) | 0.005 |
| Irrigation(Lit) | 10.34 (3.57) | 18.82 (4.76) | 0.01 |
Also, the intraoperative bleeding level did not differ significantly between the two groups (P > 0.05). However, there was a significant difference between the two groups at 24 hours after the surgery, in terms of bleeding (P = 0.005). The average amount of blood accumulated in the closed-suction drain was 36.50 mL in the fibrinogen group and 151.36 mL in the control group. The amount of irrigation solution used in the recovery room to wash the bladder until the output fluid was clear was 10.34 L in the fibrinogen group and 18.82 L in the control group, where there was a significant difference (P = 0.01).
In Table 3, hemoglobin level and hematocrit percentage were determined and compared in the two groups, three times, before the surgery and up to 12 hours afterwards. Coagulation status, in terms of PT, PTT, and INR, was compared in the two groups before and after the surgery. No statistically significant difference was observed (P > 0.05).
| Variable | Fibrinogen (N = 22) | Control (N = 22) | P Value |
|---|---|---|---|
| Hemoglobin, g/dL | |||
| Pre surgery | 12.99 (1.37) | 12.98 (1.35) | 0.99 |
| 2 hours after surgery | 12.34 (0.97) | 11.84 (0.94) | 0.09 |
| 12 hours after surgery | 12.07 (1.00) | 11.47 (1.22) | 0.08 |
| Hematocrit, % | |||
| Pre surgery | 38.28 (3.69) | 38.32 (3.45) | 0.97 |
| 2 hours after surgery | 37.43 (3.19) | 35.70 (3.24) | 0.08 |
| 12 hours after surgery | 36.23 (2.93) | 34.96 (3.48) | 0.19 |
| PT, s | |||
| Before surgery | 12.25 (0.51) | 12.23 (0.50) | 0.86 |
| After surgery | 12.20 (0.35) | 12.13 (0.34) | 0.49 |
| PTT, s | |||
| Before surgery | 33.50 (3.99) | 31.68 (6.35) | 0.26 |
| After surgery | 31.90 (2.56) | 30.63 (2.80) | 0.12 |
| INR | |||
| Before surgery | 1.02 (0.07) | 1.01 (0.06) | 0.51 |
| After surgery | 1.04 (0.06) | 1.02 (0.04) | 0.31 |
| Fibrinogen Level, g/L | |||
| Before surgery | 3.502 (1.21) | 3.504 (1.156) | 0.28 |
| After surgery | 3.458 (1.14) | 3.432 (1.095) | 0.67 |
4. Discussion
Considering the results, the patients were appropriately selected, as there was no significant difference between the two groups in terms of demographic information and underlying diseases. The amount of intraoperative bleeding was not significantly different between the two groups. In this study, using locally injected fibrinogen at the operation site at the end of surgery reduced the blood required in the postoperative period with no significant change in plasma fibrinogen concentration. The amount of blood collected in the closed-suction drain was also reduced considerably. This appeared to be the result of a local coagulation mechanism, which, with no perceivable change in plasma fibrinogen concentration, was capable of having a positive effect on patients undergoing prostatectomy surgery.
To reduce the intraoperative and postoperative bleeding in open prostatectomy, numerous actions have to be undertaken in terms of surgery and anesthesia, including administration of antifibrinolytic drugs, such as tranexamic acid and aminocaproic acid, and coagulation products, such as coagulation factors, cryoprecipitate, and fibrinogen. All of these drugs have been used in intravenous forms, and they have resulted in some complications in a number of previous studies. Intravenous injection of 2 g fibrinogen has been used by Karlsson et al. for patients undergoing open-heart surgery, for prevention of intraoperative and postoperative bleeding. They succeeded at reducing postoperative bleeding by 32%, compared to the control group. In their study, they found no clear clinical complication; however, inflammatory factors, such as D-dimer were significantly increased in patients in the fibrinogen group (21, 22). Friederich et al. used coagulation factor VII in bolus form at the beginning of surgery to control intraoperative and postoperative bleeding in open prostatectomy. They observed a decrease in bleeding level and no need for blood transfusion in patients of the intervention group. These results are consistent with those of the current study. More recent studies have evaluated the use of various compositions in topical form to obtain better results, using less drug and reducing side effects. For example, fibrin sealant formed by materials, such as fibrinogen, thrombin, and anti-fibrinolytic drugs was studied in an animal model by Alston et al. where they showed a reduction in time and level of bleeding, compared to the control group (4).
Payani et al. used topical fibrinogen on the precardium in heart surgery patients (20). Their results indicated a reduction in postoperative bleeding in coronary artery bypass surgery, and subsequently, in the postoperative period, patients had a higher hematocrit as a result of blood transfusion. The current study obtained similar results, indicating that in open prostatectomy, local injection of fibrinogen reduced postoperative bleeding. The effect may have been attributed to the activation of the coagulation process at the bleeding site, caused by fibrinogen. The mechanism may be described as follows: Fibrinogen is topically transformed to fibrin and creates a basic building block for sustainable blood clots to be formed (23). Contrary to the results obtained in these studies, Heyse et al. injected fibrinogen topically in patients undergoing knee arthroplasty surgery and observed no significant effect in terms of postoperative bleeding (24). In this case, the use of fibrinogen inside the medial knee joint space did not significantly reduce bleeding and the need for subsequent blood transfusion. In that study, fibrinogen was administered two minutes before opening the tourniquet. Lack of effect of fibrinogen compared to the control group may have been attributed to the method of cauterization applied after removal of the tourniquet. It resulted in a low bleeding level also in the control group, and no difference was observed between the control group and treatment group in terms of bleeding.
In the present study, coagulation status as determined by PT, PTT, INR, and plasma fibrinogen level was evaluated during the postoperative period and showed no difference compared to values recorded before the surgery, in either group. Payani et al. obtained the same results using topical fibrinogen in high risk patients. Coagulation parameters produced no perceptible changes in their study similar to the current study, and plasma fibrinogen concentration before and after surgery showed no significant difference (20). A slight decrease in postoperative plasma fibrinogen concentration may have resulted from intraoperative bleeding and a decrease in this coagulation factor in blood. It seems that this significant lack of change in plasma fibrinogen concentration showed the lack of systemic absorption and effectiveness of the local use of fibrinogen. Again, local use of fibrinogen resulted in no complications in either of the two studies. Contrary to the present study, Eriksen et al. evaluated intravenous administration of 2 g fibrinogen in patients experiencing a high level of bleeding, with abnormal PT and PTT, and found significantly improved levels of PT and PTT. Lack of change in PT and PTT values in the current study compared to theirs, may have resulted from local administration of fibrinogen, showing the effectiveness of local administration compared to intravenous administration of fibrinogen (25).
4.1. Conclusions
On the basis of the current results, it can be inferred that the use of fibrinogen in patients with high bleeding risk may effectively reduce bleeding level and subsequent requirement for blood transfusion, after the surgery. Meanwhile, it has no adverse effects on the coagulation status of patients, and it could be used with confidence in patients with high risk of bleeding.
Acknowledgements
References
-
1.
Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370(10):932-42. [PubMed ID: 24597866]. [PubMed Central ID: PMC4118145]. https://doi.org/10.1056/NEJMoa1311593.
-
2.
Safir I, Sanda M, Patil D, Crociani C, Hembroff L, Wei J, et al. Mp76-06 Use and Effectiveness of Pharmacologic and Non-Pharmacologic Erectile Aids among Post-Prostatectomy Prostate Cancer Survivors Seeking Treatment for Erectile Dysfunction: A Prospective, Multicenter Study. J Urol. 2016;195(4):e1008-9. https://doi.org/10.1016/j.juro.2016.02.1857.
-
3.
Boehm K, Beyer B, Tennstedt P, Schiffmann J, Budaeus L, Haese A, et al. No impact of blood transfusion on oncological outcome after radical prostatectomy in patients with prostate cancer. World J Urol. 2015;33(6):801-6. [PubMed ID: 24989847]. https://doi.org/10.1007/s00345-014-1351-0.
-
4.
Friederich PW, Henny CP, Messelink EJ, Geerdink MG, Keller T, Kurth KH, et al. Effect of recombinant activated factor VII on perioperative blood loss in patients undergoing retropubic prostatectomy: a double-blind placebo-controlled randomised trial. Lancet. 2003;361(9353):201-5. [PubMed ID: 12547542]. https://doi.org/10.1016/S0140-6736(03)12268-4.
-
5.
Nash PA, Schrepferman CG, Rowland RG, Young J, Foster RS, Birhle R, et al. The impact of pre-donated autologous blood and intra-operative isovolaemic haemodilution on the outcome of transfusion in patients undergoing radical retropubic prostatectomy. Br J Urol. 1996;77(6):856-60. [PubMed ID: 8705221].
-
6.
Pourfakhr P, Gatavi E, Gooran S, Etezadi F, Khajavi MR, Pourroustaei R, et al. Local Administration of Tranexamic Acid During Prostatectomy Surgery: Effects on Reducing the Amount of Bleeding. Nephrourol Mon. 2016;8(6). e40409. [PubMed ID: 27896241]. [PubMed Central ID: PMC5120251]. https://doi.org/10.5812/numonthly.40409.
-
7.
Ranucci M, Baryshnikova E, Crapelli GB, Rahe-Meyer N, Menicanti L, Frigiola A, et al. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. 2015;4(6). e002066. [PubMed ID: 26037084]. [PubMed Central ID: PMC4599543]. https://doi.org/10.1161/JAHA.115.002066.
-
8.
Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247-99. [PubMed ID: 15837518]. https://doi.org/10.1016/S0065-3233(05)70008-5.
-
9.
Hayakawa M, Sawamura A, Gando S, Kubota N, Uegaki S, Shimojima H, et al. Disseminated intravascular coagulation at an early phase of trauma is associated with consumption coagulopathy and excessive fibrinolysis both by plasmin and neutrophil elastase. Surgery. 2011;149(2):221-30. [PubMed ID: 20655560]. https://doi.org/10.1016/j.surg.2010.06.010.
-
10.
Martini WZ, Chinkes DL, Pusateri AE, Holcomb JB, Yu YM, Zhang XJ, et al. Acute changes in fibrinogen metabolism and coagulation after hemorrhage in pigs. Am J Physiol Endocrinol Metab. 2005;289(5):E930-4. [PubMed ID: 15956050]. https://doi.org/10.1152/ajpendo.00137.2005.
-
11.
Gerlach R, Raabe A, Zimmermann M, Siegemund A, Seifert V. Factor XIII deficiency and postoperative hemorrhage after neurosurgical procedures. Surg Neurol. 2000;54(3):260-4. discussion 264-5. [PubMed ID: 11118574].
-
12.
Gerlach R, Tolle F, Raabe A, Zimmermann M, Siegemund A, Seifert V. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke. 2002;33(6):1618-23. [PubMed ID: 12053001].
-
13.
Godje O, Gallmeier U, Schelian M, Grunewald M, Mair H. Coagulation factor XIII reduces postoperative bleeding after coronary surgery with extracorporeal circulation. Thorac Cardiovasc Surg. 2006;54(1):26-33. [PubMed ID: 16485185]. https://doi.org/10.1055/s-2005-872853.
-
14.
Fenger-Eriksen C, Ingerslev J, Sorensen B. Fibrinogen concentrate--a potential universal hemostatic agent. Expert Opin Biol Ther. 2009;9(10):1325-33. [PubMed ID: 19645632]. https://doi.org/10.1517/14712590903193051.
-
15.
Kreuz W, Meili E, Peter-Salonen K, Haertel S, Devay J, Krzensk U, et al. Efficacy and tolerability of a pasteurised human fibrinogen concentrate in patients with congenital fibrinogen deficiency. Transfus Apher Sci. 2005;32(3):247-53. [PubMed ID: 15919240]. https://doi.org/10.1016/j.transci.2004.08.003.
-
16.
Levy JH, Goodnough LT. How I use fibrinogen replacement therapy in acquired bleeding. Blood. 2015;125(9):1387-93. [PubMed ID: 25519751]. https://doi.org/10.1182/blood-2014-08-552000.
-
17.
Lowe GD, Rumley A, Woodward M, Morrison CE, Philippou H, Lane DA, et al. Epidemiology of coagulation factors, inhibitors and activation markers: the Third Glasgow MONICA Survey. I. Illustrative reference ranges by age, sex and hormone use. Br J Haematol. 1997;97(4):775-84. [PubMed ID: 9217176].
-
18.
Agger P, Langhoff J, Smerup MH, Hasenkam JM. Comparison between TachoComb and TachoSil for surgical hemostasis in arterial bleeding: an animal experimental study. J Trauma. 2010;68(4):838-42. [PubMed ID: 20386279]. https://doi.org/10.1097/TA.0b013e3181b1388c.
-
19.
Alizadeh Ghavidel A, Mirmesdagh Y, Samiei N, Gholampour Dehaki M. Haemostatic Role of TachoSil Surgical Patch in Cardiac Surgery. J Cardiovasc Thorac Res. 2014;6(2):91-5. [PubMed ID: 25031823]. [PubMed Central ID: PMC4097858]. https://doi.org/10.5681/jcvtr.2014.020.
-
20.
Payani N, Foroughi M, Rajaei S, Dabbagh A. Effect of local fibrinogen administration on postoperative bleeding in coronary artery bypass graft patients. J Cell Mol Anesthes. 2016;1(1):23-7.
-
21.
Han M, Partin AW, Wein AJ, Kavoussi LR, Novick AC, Peters CA. Campbell-Walsh urology. 2007.
-
22.
Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Nilsson S, Jeppsson A. Plasma fibrinogen level, bleeding, and transfusion after on-pump coronary artery bypass grafting surgery: a prospective observational study. Transfusion. 2008;48(10):2152-8. [PubMed ID: 18657083]. https://doi.org/10.1111/j.1537-2995.2008.01827.x.
-
23.
Capraro L. Transfusion practices in elective surgical procedures in finnish hospitals. 2001.
-
24.
Heyse TJ, Haas SB, Drinkwater D, Lyman S, Kim HJ, Kahn BA, et al. Intraarticular fibrinogen does not reduce blood loss in TKA: a randomized clinical trial. Clin Orthop Relat Res. 2014;472(1):272-6. [PubMed ID: 23657879]. [PubMed Central ID: PMC3889440]. https://doi.org/10.1007/s11999-013-3036-1.
-
25.
Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, Ingerslev J, Sorensen B. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth. 2008;101(6):769-73. [PubMed ID: 18818192]. https://doi.org/10.1093/bja/aen270.