Abstract
Background:
Labor pain is a severe pain, and intrathecal opioid injection is one of the analgesia methods to reduce it.Objectives:
We assessed the effects of intrathecal Fentanyl and Sufentanil on the onset, duration, and quality of analgesia for labor analgesia.Methods:
In this double-blind, randomized clinical trial, 54 healthy nulliparous women 18 - 45 years in the active phase of labor who were requesting labor analgesia were enrolled in two groups fentanyl (F) and sufentanil (S). Patients received 75 µg fentanyl or 7.5 µg sufentanil intrathecally in the fentanyl group (n = 27) and the sufentanil group (n = 27), respectively. Pain relief, onset, duration of analgesia, hemodynamic parameters, patients' satisfaction, and neonatal Apgar score were assessed in this study. Data were analyzed by using SPSS16.Results:
There were no significant differences between the groups in terms of demographic and hemodynamic parameters. The onset time of analgesia was 5.6 ± 4.3 and 3.6 ± 2.1 minutes, in the sufentanil and fentanyl groups, respectively (P = 0.037). The duration of analgesia was higher in patients who received sufentanil than those who received fentanyl (113 ± 45 vs. 103 ± 22 minutes (P = 0.629)). The pain score in the Fentanyl group was significantly lower at 5, 10, and 15 minutes after spinal analgesia (P < 0.05). The sedation score at 1 and 5 minutes was significantly higher in the fentanyl group than the sufentanil group (P < 0.05). The frequency and severity of pruritus and satisfaction rate in the fentanyl group were significantly higher than the sufentanil group.Conclusions:
Intrathecal fentanyl and sufentanil have a similar analgesic effect on labor. Fentanyl is associated with a faster onset of analgesia and more satisfaction, while sufentanil has longer analgesia.Keywords
Analgesia Fentanyl Sufentanil Intrathecal Injection Labor Pain
1. Background
The labor pain is the most severe pain that a woman may ever experience in her life. Therefore, women fear vaginal labor (1, 2). Dilation of the inferior segment of the uterus and cervix at the first stage of labor and dilation and ischemia of the perineal and vaginal tissues at the second stage causes this pain (1, 3). Physical and psychological factors such as age, parity, fetus size, fetus position, and so on, determine the severity of the pain and its duration (3, 4). There are various methods to mitigate labor pain, including pharmacological and non-pharmacological methods. Pharmacological methods involve using Entonox, systemic opioids, and regional methods (5, 6). Regional analgesia involves spinal, epidural, and combined spinal-epidural methods that apply to induce analgesia in labor (7, 8). In spinal analgesia, single-dose injection of opioids alone or in combination with a lower dose of anesthetics to the subarachnoid space causes rapid and effective analgesia during labor (8, 9). Hypotension is the most common complication of spinal anesthesia and analgesia (10, 11). Administration of opiates alone, which does not have any effect on the sympathetic block, is a common method to decrease the incidence of hypotension and fetal complications (12). Intrathecal opiates are a popular choice for labor (13). Fentanyl and sufentanil are fat-soluble opioids used in spinal anesthesia and analgesia (14, 15). Most of the previous studies conducted on these drugs investigated them in combination with low doses of local anesthetics in surgical operations, while few studies compared the intrathecal effects of these opioids on the onset, duration, and quality of analgesia for labor (13-15).
2. Objectives
The current double-blind, randomized study aimed to compare the effects of intrathecal injection fentanyl and sufentanil on the onset, duration, and quality of analgesia for labor.
3. Methods
3.1. Study Population
After approval by the Ethical Committee of the Hamadan University of Medical Sciences (code: IR.UMSHA.REC.1397.223 & IRCT20120915010841N10) and taking written informed consent from participants, this double-blind, randomized clinical trial was conducted at Fatemieh Hospital of Hamadan in 2018. Data were collected using a researcher-made checklist following the research objectives and variables. Subjects were selected using the convenience sampling method, and the sample size was estimated to be 54 (27 patients in each group). All patients were nulliparous, 18 - 45 years old with American Society of Anesthesiologists physical status I or II, at term gestation (37 - 40 weeks), candidate for vaginal delivery under spinal analgesia and in active labor, with a cervical dilation more than 5 cm when requesting labor analgesia.
Exclusion criteria were patients’ refusal to continue the participation, reduction in consciousness level, spinal failure, contraindications to regional anesthesia, receiving narcotic analgesics 24 hours before hospitalization, and allergy to opioids.
3.2. Study Design
Using block randomization with a block size of six for each group, patients were randomly allocated to one of the sufentanil (S) and fentanyl (F) groups. We chose a block randomly, and the first six treatments were allocated according to the block. Then a new block was chosen randomly, and the next six treatments were allocated. The process continued until the allocation of all subjects to a group. Before spinal analgesia, systolic and diastolic blood pressure, heart rate (HR), respiratory rate (RR), and blood oxygen saturation (SPO2) were measured (by non-invasive blood pressure and ECG monitoring, Saadat, Made in Iran) and recoded. Pain score, fetal heart rate, and sedation score before spinal analgesia were recorded in the checklist. Then, the spinal analgesia was performed using the Quinke no.: 26 needle in the L3-L4 or L4-L5 space in the sitting position. The F group received 1.5 mL (75 µg) fentanyl (Feniject, Aburehan, Iran), and the group S received 1.5 mL (7.5 µg) sufentanil (Sufiject, Aburehan, Iran). All intrathecal injections were administered in a 1.5 mL volume. Besides, similar syringes were prepared by an anesthetic nurse according to the block randomization list and were intrathecally injected by an anesthesiologist who had no awareness about the type of drug. After spinal analgesia, pain score (measured using Visual Analog scale (VAS)), blood pressure (systolic, diastolic), HR, RR, fetal heart rate (FHR), SPO2, and sedation score (by Ramsay scale) were assessed every 5 minutes for three times and then were assessed every 15 minutes up to 135 minutes. FHR (measured by fetal cardiac monitoring device), pain relapse time, frequency and severity of pruritus (using the VAS), nausea and vomiting, shivering, patients’ satisfaction, Apgar of the neonates (first and fifth minutes), duration of the first and second stages of labor and the rate of cesarean section were recorded in the checklist. When the analgesic effect was over, and the patient's pain returned (VAS ≥ 6), drugs such as Pethidine (intravenously) or Entonox were administered, depending on the patient’s condition and the progress of the delivery. The pain intensity was assessed using the VAS. In the current study, VAS was measured by using a 10 cm ruler, the score was determined by measuring the distance on the 10‐cm line between the zero and the patient’s mark, therefore scores ranged from 0 - 10, “no pain” on the right side (i.e.) and “worst possible pain” on the left side (i.e. 10). To assess the severity of pruritus, the VAS approach was used, the same as the methodology used for assessing the pain. In this way, patients mark a number on a 10cm ruler based on the severity of their pruritus. The following VAS category was proposed: 0 = no pruritus, > 0- < 4 points = mild pruritus, ≥ 4- < 7 points = moderate pruritus, ≥ 7-< 9 points = severe pruritus, and ≥ 9 points = very severe pruritus. Patients’ satisfaction (subjective assessment of the quality of neuraxial labor analgesia) was defined as a numerical rating scale as a percentage (0 to 100%) reported by the parturient at post-delivery in recovery. Parturients with reported satisfaction lower than 80%, 80% - 90%, and greater than 90% were considered as not satisfied, satisfied, and very satisfied, respectively. Sedation was assessed by Ramsay sedation scale as follows (Table 1):
Ramsay Sedation Scale
| Score | Response |
|---|---|
| 1 | Anxious, or restless, or both |
| 2 | Cooperative, orientated, and tranquil |
| 3 | Responding to commands |
| 4 | Brisk response to stimulus |
| 5 | Sluggish response to stimulus |
| 6 | No response to stimulus |
3.3. Statistical Analysis
Data were analyzed using SPSS software version 16. To compare pain, Apgar, and sedation scores, and duration of the first and second stages of labor, t-test and Mann-Whitney tests were used. Chi-square and Fisher’s exact tests were used to compare the frequency of complications, fetus bradycardia, and satisfaction. A P value of less than 0.05 was considered statistically significant.
4. Results
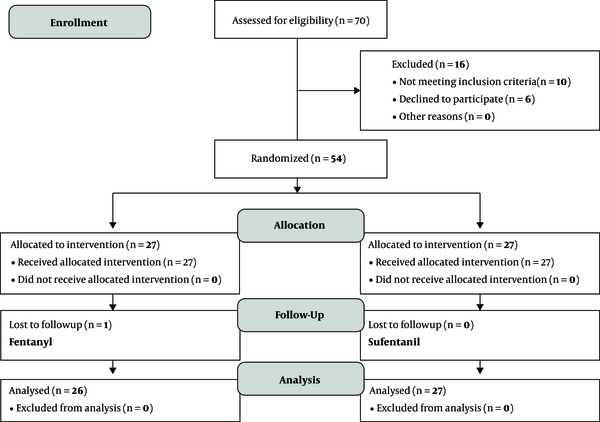
In the current clinical trial which aimed to compare the intrathecal injection of fentanyl and sufentanil on the onset, duration, and quality of labor analgesia under the spinal block, 53 patients in two groups (27 in each group) were evaluated. One patient in the fentanyl group was excluded due to a lack of cooperation in answering questions (Figure 1). The value of mean age in sufentanil and fentanyl groups was 23.8 ± 4.1 and 25.9 ± 4.9 years, respectively (P = 0.094), and two groups were homogeneous in terms of age. According to the results, no significant difference was observed in terms of baseline systolic and diastolic blood pressure, HR, RR, SPO2, FHR, sedation, and pain scores between the two groups. Both groups were homogeneous in terms of the aforementioned variables. The results also revealed that the analgesia began significantly sooner in the fentanyl group (3.6 ± 2.1 minutes) than sufentanil (5.6 ± 4.3 minutes) (P = 0.037). Besides, the duration of analgesia was higher in the sufentanil group than the fentanyl group (113.1 ± 45.4 vs 103.6 ± 22.9 minutes), but there was no significant difference between the two groups (P = 0.624) (Table 2). Based on the results, the mean pain score of the fentanyl group at 5,10 and 15 minutes was significantly lower than that of the sufentanil group (P < 0.05). However, the pain score was not significantly different between the two groups at 30 to 135 minutes (Table 3). Comparison of sedation score in fentanyl and sufentanil groups revealed that, except for the scores related to the first 5 minutes, the sedation scores of sufentanil group were significantly lower than fentanyl group (P < 0.05), and no significant difference was found between the two groups in the rest of measurements (Table 3). The results also revealed that there was no significant difference in terms of nausea, shivering, and rate of the cesarean section between two groups. However, the frequency and severity of pruritus were significantly higher in the fentanyl group. So that, the severity of pruritus was moderate (5.1 ± 1.7) in the Fentanyl group and mild (3.7 ± 0.8) in the sufentanil group (Table 2). While there was no significant difference between the two groups in terms of mean systolic and diastolic blood pressure, HR, and FHR at all measured times (Table 4). Also, two groups did not differ at all times in terms of RR and SPO2. Indeed, there was no significant difference in terms of frequency of fetus bradycardia in two groups at different times (P > 0.05). Mean Apgar scores were similar in both groups at 1 and 5 minutes (8.9 ± 0.1 and 9.9 ± 0.1, respectively) and there was no significant difference between the two groups in this regard (P = 0.978). In addition, there was no significant difference in sufentanil and Fentanyl groups in terms of the mean duration of the first stage (52.4 ± 33.6 vs 46.6 ± 33.4 min) and the second stage (26.5 ± 19.4 and 26.4 ± 18.5 min) of labor and pain score at the end of those stages (Table 5). In the current study, the level of satisfaction in the Fentanyl group was significantly higher than the sufentanil group (P = 0.011).
Flowchart of the trial (consort chart)
The Onset and Duration of Analgesia and Frequency of Maternal Side Effects in Sufentanil and Fentanyl Groupsa
| Variables | S Group (N = 27) | F Group (N = 26) | P Value |
|---|---|---|---|
| Onset of analgesia, min | 5.64 ± 4.35 | 3.66 ± 2.13 | 0.037b |
| Duration of analgesia, min | 113.17 ± 45.43 | 103.67 ± 22.95 | 0.624 |
| Frequency of pruritus | 11 (40.7) | 18 (69.2) | 0.037b |
| Severity of pruritus (VAS) | 3.70 ± 0.82 | 5.12 ± 1.73 | 0.027b |
| Frequency of shivering | 4 (14.8) | 2 (7.7) | 0.669 |
| Severity of shivering | 2.25 ± 1.71 | 1.00 ± 1.41 | 0.533 |
| Frequency of Nausea | 1 (3.7) | 1 (3.8) | 0.978 |
| Caesarian section | 1 (9.1) | 0 (0) | 0.379 |
Comparison of Pain and Sedation Score in Sufentanil and Fentanyl Groups Based on Measurement Timea
| Measurement Time | Pain Score | P Value | Sedation Score | P Value | ||
|---|---|---|---|---|---|---|
| S Group | >F Group | S Group | F Group | |||
| Minute 1 | 9.00 ± 2.00 | 8.00 ± 6.25 | 0.084 | 1.34 ± 0.48 | 1.65 ± 0.48 | 0.028b |
| Minute 5 | 4.5 ± 7.75 | 0.00 ± 6.25 | < 0.001b | 1.77 ± 0.51 | 2.23 ± 0.43 | 0.001b |
| Minute 10 | 2.50 ± 5.75 | 0.00 ± 3.75 | < 0.001b | 2.00 ± 0.49 | 2.31 ± 0.47 | 0.30 |
| Minute 15 | 1.50 ± 5.25 | 0.00 ± 0.75 | 0.010b | 2.08 ± 0.39 | 2.27 ± 0.45 | 0.112 |
| Minute 30 | 1.50 ± 3.00 | 0.00 ± 0.75 | 0.184 | 2.08 ± 0.40 | 2.27 ± 0.45 | 0.134 |
| Minute 45 | 1.00 ± 2.75 | 0.00 ± 0.75 | 0.358 | 2.14 ± 0.36 | 2.31 ± 0.48 | 0.161 |
| Minute 60 | 1.00 ± 2.75 | 0.00 ± 1.50 | 0.346 | 2.19 ± 0.40 | 2.31 ± 0.60 | 0.495 |
| Minute 75 | 2.50 ± 2.50 | 0.00 ± 2.25 | 0.697 | 2.07 ± 0.61 | 2.22 ± 0.67 | 0.585 |
| Minute 90 | 2.0 ± 4.25 | 0.00 ± 2.25 | 0.296 | 2.00 ± 0.58 | 2.12 ± 0.64 | 0.649 |
| Minute 105 | 2.00 ± 4.25 | 0.00 ± 3.00 | 0.855 | 2.14 ± 0.69 | 1.80 ± 0.45 | 0.356 |
| Minute 120 | 2.50 ± 5.50 | 2.50 ± 6.25 | 0.736 | 2.17 ± 0.75 | 1.75 ± 0.50 | 0.363 |
| Minute 135 | 4.00 ± 6.50 | 2.50 ± 4.75 | 0.914 | 2.00 ± 0.82 | 2.00 ± 1.00 | 1.00 |
Comparison of Systolic and Diastolic Blood Pressure, HR and FHR in Sufentanil and Fentanyl Groups Based on Measurement Timea
| Measurement Time | Systolic Blood Pressure, mmHg | Diastolic Blood Pressure, mmHg | Heart Rate, bpm | Fetal Heart Rate, bpm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S Group | F Group | P Value | S Group | F Group | P Value | S Group | F Group | P Value | S Group | F Group | P Value | |
| Before intervention | 121.81 ± 12.23 | 118.58 ± 15.80 | 0.407 | 70.70 ± 14.81 | 73.92 ± 10.71 | 0.376 | 96.11 ± 16.82 | 91.34 ± 22.29 | 0.383 | 128 ± 0.44 | 132 ± 0.46 | 1.00 |
| Minute 1 | 115.03 ± 24.57 | 117.76 ± 11.92 | 0.611 | 70.74 ± 14.81 | 73.92 ± 10.71 | 0.350 | 89.74 ± 12.99 | 94.65 ± 17.23 | 0.246 | 134 ± 0.48 | 165 ± 0.48 | 0.028b |
| Minute 5 | 113.18 ± 7.39 | 112.92 ± 11.07 | 0.919 | 64.88 ± 9.55 | 69.53 ± 12.60 | 0.135 | 92.59 ± 18.70 | 88.50 ± 15.64 | 0.392 | 177 ± 0.51 | 123 ± 0.43 | 0.001b |
| Minute 10 | 115.11 ± 11.06 | 116.26 ± 10.38 | 0.696 | 66.66 ± 12.37 | 69.73 ± 13.81 | 0.399 | 90.81 ± 12.14 | 88.53 ± 16.59 | 0.570 | 130 ± 0.49 | 161 ± 0.47 | 0.30 |
| Minute 15 | 115.37 ± 12.20 | 116.46 ± 14.32 | 0.766 | 65.74 ± 10.90 | 71.80 ± 15.37 | 0.103 | 92.74 ± 12.72 | 91.53 ± 16.04 | 0.763 | 118 ± 0.39 | 127 ± 0.45 | 0.112 |
| Minute 30 | 115.65 ± 10.68 | 115.34 ± 9.44 | 0.916 | 67.46 ± 9.46 | 68.82 ± 11.12 | 0.645 | 94.69 ± 13.56 | 88.78 ± 11.87 | 0.114 | 118 ± 0.40 | 137 ± 0.45 | 0.134 |
| Minute 45 | 113.86 ± 12.17 | 106.78 ± 19.63 | 0.167 | 63.81 ± 9.25 | 66.20 ± 12.61 | 0.487 | 92.72 ± 12.79 | 87.45 ± 12.32 | 0.182 | 114 ± 0.36 | 131 ± 0.48 | 0.161 |
| Minute 60 | 114.70 ± 10.22 | 111.41 ± 7.4 | 0.292 | 64.58 ± 9.70 | 63.11 ± 8.63 | 0.644 | 93.35 ± 12.65 | 86.00 ± 13.77 | 0.115 | 119 ± 0.40 | 131 ± 0.60 | 0.495 |
| Minute 75 | 113.35 ± 11.84 | 111.00 ± 10.66 | 0.622 | 65.28 ± 12.13 | 64.72 ± 16.93 | 0.924 | 94.14 ± 13.85 | 85.70 ± 10.76 | 0.122 | 117 ± 0.61 | 132 ± 0.67 | 0.585 |
| Minute 90 | 120.92 ± 16.99 | 115.42 ± 13.20 | 0.469 | 69.15 ± 14.31 | 64.77 ± 13.21 | 0.476 | 93.07 ± 11.59 | 85.87 ± 10.73 | 0.172 | 113 ± 0.58 | 126 ± 0.64 | 0.649 |
| Minute 105 | 122.14 ± 14.22 | 114.50 ± 13.69 | 0.347 | 66.00 ± 12.30 | 67.50 ± 17.22 | 0.851 | 95.28 ± 14.18 | 91.66 ± 14.16 | 0.655 | 144 ± 0.69 | 154 ± 0.45 | 0.356 |
| Minute 120 | 121.00 ± 15.16 | 112.20 ± 10.84 | 0.307 | 64.16 ± 14.79 | 64.57 ± 12.90 | 0.959 | 97.33 ± 17.64 | 92.20 ± 14.35 | 0.615 | 137 ± 0.75 | 159 ± 0.50 | 0.363 |
| Minute 135 | 126.50 ± 14.57 | 114.25 ± 12.81 | 0.254 | 69.50 ± 13.12 | 70.60 ± 17.68 | 0.921 | 99.50 ± 26.80 | 93.75 ± 16.23 | 0.691 | 139 ± 0.82 | 141 ± 0.1 | 1.00 |
Comparison of Mean Duration and Pain Score of the First and Second Stages of Labor in Sufentanil and Fentanyl Groupsa
| Stages of Labor | Duration, min | Pain Score, vas | ||||
|---|---|---|---|---|---|---|
| S Group | F Group | P Value | S Group | F Group | P Value | |
| First stage | 52.45 ± 33.63 | 46.65 ± 33.40 | 0.074 | 1.73 ± 2.10 | 1.27 ± 2.01 | 0.294 |
| Second stage | 26.51 ± 19.49 | 26.46 ± 18.52 | 0.971 | 2.54 ± 3.06 | 2.20 ± 2.78 | 0.699 |
5. Discussion
The results of the current study showed that the onset time and duration of analgesia were significantly higher for the sufentanil. Nelson and colleagues compared the effect of intrathecal fentanyl and sufentanil on labor analgesia and reported that the duration of analgesia with sufentanil was longer than fentanyl (13). In a similar study, Farzi et al. (16) added fentanyl (25 µg) and sufentanil (2.5 µg) to intrathecal Bupivacaine in spinal anesthesia for C/S and found that intrathecal fentanyl had a similar duration of analgesia like sufentanil with a faster return of motor block and ambulation. In another study, Lilker et al. (17) compared the analgesic effect of epidural sufentanil (5 µg) and fentanyl (25 µg) and reported that epidural fentanyl and sufentanil with low-dose bupivacaine improved the a nalgesia in terms of both onset and duration. Moreover, Akkamahadevi et al. (18) compared the effect of sufentanil (5 µg) and fentanyl (25 µg) combined with spinal-epidural analgesia (CSE) and found that Sufentanil induced longer analgesia than fentanyl. The results of the above studies are consistent with the current study. In a study conducted by Connelly et al. (19), the duration of analgesia in the epidural fentanyl group was longer than the sufentanil group, but the difference was not statistically significant. Rolfseng et al. (20) compared the effects and side effects of epidural fentanyl and sufentanil on the labor and reported no significant difference in the duration of analgesia and side effects between the two drugs. The results of the present study are consistent with those of Lilker et al. (17), Akkamahadevi et al. (18), Nelson et al. (13), Farzi et al. (16), and Rolfseng et al. (20). However, the results of the current study are contradicted with the results of the study conducted by Connelly concerning the duration of analgesia (19). It might be due to differences in the sample size, prescription method, or dose of drugs. In the present study, the pain score in both groups decreased compared to the pre-intervention. The pain score in the fentanyl group at 5, 10, and 15 minutes was significantly lower than the sufentanil group (because of the faster onset of analgesia in the fentanyl group), but there was no significant difference between the two groups at 15 to 135 minutes. Studies conducted by Lilker et al. (17), Akkamahadevi et al. (18), Farzi et al. (16), and Nelson et al. (13) also reported that fentanyl and sufentanil reduced labor pain. Moreover, no significant difference was reported between two groups in terms of nausea as well as the frequency and severity of shivering. Lilker et al. (17) and Connelly et al. (19) conducted two studies and found no significant difference between fentanyl and sufentanil groups in terms of nausea and vomiting. However, in the current study, the frequency and severity of pruritus in the fentanyl group were significantly higher than the sufentanil group. These results are consistent with those reported in the study conducted by Farzi et al. (16) which showed that the frequency of pruritus in the fentanyl group was higher than sufentanil and placebo groups. But they are contradicted with the results of Dahlgren et al. (21), that reported the frequency of pruritus was higher in the sufentanil group than fentanyl in C/S. Also, there are differences between the results of the current study and the results of Farzi et al. (22) concerning the higher frequency of pruritus with fentanyl.
In the current study, there was no significant difference in the frequency of fetus bradycardia between the two groups. These results are consistent with those of Nelson et al. (13). Moreover, there was no significant difference in Apgar score at 1 and 5 minutes in two groups. Our results are in line with the results of studies conducted by Lilker et al. (17), Akkamahadevi et al. (18), and Dahlgren et al. (21). However, in the current study, only one woman in the sufentanil group had a cesarean section, and there was no significant difference between sufentanil and fentanyl groups. No significant difference was found between two groups in terms of the mean duration of the first and second stages of labor and pain scores at the end of those stages. These results are consistent with the results of the Connelly et al. study (19). However, in contrast to the results of the current study, a study conducted by Rolfseng et al. (20) reported that the duration of the second stage was significantly higher in the sufentanil group than the fentanyl group. However, the researchers stated that this difference was clinically non-significant due to the small sample size in their study and the small differences between the studied groups. In the present study, the sedation score of the sufentanil group was significantly lower than the fentanyl group at 1 and 5 minutes that this was due to the faster onset of analgesia in the fentanyl group, which makes the patient calmer. These results are consistent with the results of the Rolfseng et al. study (20). In the present study, the satisfaction level in the Fentanyl group was significantly higher than the sufentanil group, which might be due to the faster onset of analgesia in the fentanyl group. In the Lilker et al. (17) study, no significant difference was observed between sufentanil and fentanyl groups in terms of the satisfaction level. It might be due to differences in sample size, method of administration, or the dose of drugs.
5.1. Conclusions
Intrathecal fentanyl and sufentanil have a similar analgesic effect on labor. Fentanyl is associated with a faster onset of analgesia and more satisfaction while sufentanil has longer analgesia.
Acknowledgements
References
-
1.
Makvandi S, Mirzaiinajmabadi K, Tehranian N, Esmily H, Mirteimoori M. The effect of normal physiologic childbirth on labor pain relief: An interventional study in mother-friendly hospitals. Maedica (Buchar). 2018;13(4):286-93. [PubMed ID: 30774727]. [PubMed Central ID: PMC6362879]. https://doi.org/10.26574/maedica.2018.13.4.286.
-
2.
Khwepeya M, Lee GT, Chen SR, Kuo SY. Childbirth fear and related factors among pregnant and postpartum women in Malawi. BMC Pregnancy Childbirth. 2018;18(1):391. [PubMed ID: 30285754]. [PubMed Central ID: PMC6171200]. https://doi.org/10.1186/s12884-018-2023-7.
-
3.
Jones L, Othman M, Dowswell T, Alfirevic Z, Gates S, Newburn M, et al. Pain management for women in labour: An overview of systematic reviews. Cochrane Database Syst Rev. 2012;(3). CD009234. [PubMed ID: 22419342]. [PubMed Central ID: PMC7132546]. https://doi.org/10.1002/14651858.CD009234.pub2.
-
4.
Lederman RP, McCann DS, Work BJ, Huber MJ. Endogenous plasma epinephrine and norepinephrine in last-trimester pregnancy and labor. Am J Obstet Gynecol. 1977;129(1):5-8. [PubMed ID: 900169]. https://doi.org/10.1016/0002-9378(77)90809-2.
-
5.
Gupta S, Partani S. Neuraxial techniques of labour analgesia. Indian J Anaesth. 2018;62(9):658-66. [PubMed ID: 30237590]. [PubMed Central ID: PMC6144556]. https://doi.org/10.4103/ija.IJA_445_18.
-
6.
Manouchehrian N, Bakhshaei MH. Nitrous oxide effect on relieving anxiety and pain in parturients under spinal anesthesia for caesarean section. Anesth Pain Med. 2014;4(2). e16662. [PubMed ID: 24977119]. [PubMed Central ID: PMC4071269]. https://doi.org/10.5812/aapm.16662.
-
7.
Singh SK, Yahya N, Misiran K, Masdar A, Nor NM, Yee LC. Combined spinal-epidural analgesia in labour: Its effects on delivery outcome. Braz J Anesthesiol. 2016;66(3):259-64. [PubMed ID: 27108822]. https://doi.org/10.1016/j.bjane.2014.09.006.
-
8.
Minty RG, Kelly L, Minty A, Hammett DC. Single-dose intrathecal analgesia to control labour pain: Is it a useful alternative to epidural analgesia? Can Fam Physician. 2007;53(3):437-42. [PubMed ID: 17872679]. [PubMed Central ID: PMC1949078].
-
9.
Goodman SR, Kim-Lo SH, Ciliberto CF, Ridley DM, Smiley RM. Epinephrine is not a useful addition to intrathecal fentanyl or fentanyl-bupivacaine for labor analgesia. Reg Anesth Pain Med. 2002;27(4):374-9. [PubMed ID: 12132061]. https://doi.org/10.1053/rapm.2002.33283.
-
10.
Manouchehrian N, Bashar FR, Arab M. Efficacy of intrathecal injection rate of bupivacaine 0.5 on sensory and motor block. J Babol Univ Med Sci. 2014;16(9):21-8.
-
11.
Ben-David B, Miller G, Gavriel R, Gurevitch A. Low-dose bupivacaine-fentanyl spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25(3):235-9. [PubMed ID: 10834776].
-
12.
Norris MC, Arkoosh VA. Spinal opioid analgesia for labor. Int Anesthesiol Clin. 1994;32(2):69-81. [PubMed ID: 7914885].
-
13.
Nelson KE, Rauch T, Terebuh V, D'Angelo R. A comparison of intrathecal fentanyl and sufentanil for labor analgesia. Anesthesiology. 2002;96(5):1070-3. [PubMed ID: 11981144]. https://doi.org/10.1097/00000542-200205000-00007.
-
14.
D'Angelo R, Anderson MT, Philip J, Eisenach JC. Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology. 1994;80(6):1209-15. [PubMed ID: 8010467]. https://doi.org/10.1097/00000542-199406000-00007.
-
15.
Vercauteren MP, Jacobs S, Jacquemyn Y, Adriaensen HA. Intrathecal labor analgesia with bupivacaine and sufentanil: The effect of adding 2.25 microg epinephrine. Reg Anesth Pain Med. 2001;26(5):473-7. [PubMed ID: 11561270]. https://doi.org/10.1053/rapm.2001.24406.
-
16.
Farzi F, Mirmansouri A, Naderi Nabi B, Atrkar Roushan Z, Ghazanfar Tehran S, Nematollahi Sani M, et al. Comparing the effect of adding fentanyl, sufentanil, and placebo with intrathecal bupivacaine on duration of analgesia and complications of spinal anesthesia in patients undergoing cesarean section. Anesth Pain Med. 2017;7(5). e12738. [PubMed ID: 29696107]. [PubMed Central ID: PMC5903220]. https://doi.org/10.5812/aapm.12738.
-
17.
Lilker S, Rofaeel A, Balki M, Carvalho JC. Comparison of fentanyl and sufentanil as adjuncts to bupivacaine for labor epidural analgesia. J Clin Anesth. 2009;21(2):108-12. [PubMed ID: 19329014]. https://doi.org/10.1016/j.jclinane.2008.06.027.
-
18.
Akkamahadevi P, Srinivas H, Siddesh A, Kadli N. Comparision of efficacy of sufentanil and fentanyl with low-concentration bupivacaine for combined spinal epidural labour analgesia. Indian J Anaesth. 2012;56(4):365-9. [PubMed ID: 23087459]. [PubMed Central ID: PMC3469915]. https://doi.org/10.4103/0019-5049.100819.
-
19.
Connelly NR, Parker RK, Vallurupalli V, Bhopatkar S, Dunn S. Comparison of epidural fentanyl versus epidural sufentanil for analgesia in ambulatory patients in early labor. Anesth Analg. 2000;91(2):374-8. [PubMed ID: 10910851]. https://doi.org/10.1097/00000539-200008000-00026.
-
20.
Rolfseng OK, Skogvoll E, Borchgrevink PC. Epidural bupivacaine with sufentanil or fentanyl during labour: A randomized, double-blind study. Eur J Anaesthesiol. 2002;19(11):812-8. [PubMed ID: 12442931]. https://doi.org/10.1017/s026502150200131x.
-
21.
Dahlgren G, Hultstrand C, Jakobsson J, Norman M, Eriksson EW, Martin H. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg. 1997;85(6):1288-93. [PubMed ID: 9390596]. https://doi.org/10.1097/00000539-199712000-00020.
-
22.
Farzi F, Mirmansouri A, Forghanparast K, Heydarzadeh A, Abdollahzadeh M, Jahanyar Moghadam F. Addition of intrathecal fentanyl or meperidine to lidocaine and epinephrine for spinal anesthesia in elective cesarean delivery. Anesth Pain Med. 2014;4(1). e14081. [PubMed ID: 24701418]. [PubMed Central ID: PMC3961034]. https://doi.org/10.5812/aapm.14081.