Abstract
Objectives:
Estrogen is one of the important factors in the progression of breast cancer. Cytochrome P450 Superfamily (CYPs) plays an important role in the metabolism of estrogen. The CYP1A1 gene has been shown to be involved in the metabolism of estradiol. Several studies have reported a significant association between CYP1A1 polymorphisms and breast cancer.Methods:
In the present work, the association between CYP1A1 polymorphism (Thr461Asn) and breast cancer in Guilan province was investigated. The blood samples were obtained from 120 patients and 80 controls. After DNA extraction, specific primers were adapted to amplify the specific fragment using restriction fragment length polymorphism-polymerase chain reaction (RFLP-PCR) and the results were examined through gel-electrophoresis. The product of amplification was digested with restriction enzyme BsmA1.Results:
Genotype distributions of the CYP1A1 gene showed no significant difference between patients and controls (P = 0.12, Allele A in patients 0.16, in controls 0.10, Allele C in patients 0.84, and controls 0.9, respectively).Conclusions:
The results from this study suggest that the Thr461Asn polymorphism of CYP1A1 gene may not be associated with the risk of breast cancer in the population of Guilan province. Further studies are needed to confirm the role of CYP1A1 gene in breast cancer.Keywords
1. Background
Breast cancer incidence has increased steadily worldwide in the recent years and it remains the major cause of cancer-related death among females (1). Genetic factors and individual lifestyle habits could both influence the risk of breast cancer (2). Studies have shown that the risk of breast cancer is associated with a combination of factors, such as older age, early menstrual period, late or no pregnancy, starting menopause after age of 55, not being physically active, having dense breasts, being overweight, using combination hormone therapy, previous treatment using radiation therapy, personal history of breast cancer, smoking, and drinking alcohol. About 5% to 10% of breast cancers are thought to be hereditary, caused by abnormal genes passed from parents to their child and 90% to 95% of breast cancer cases do not develop due to an abnormal gene. Instead, they are caused by a female’s genetic makeup combined with environmental factors. This type of breast cancer is called sporadic (3). Under the polygenic model, a combination of multiple low risk genes with variants across the genome could produce susceptibility to the disease. Several genome-wide association studies (GWASs) identified multiple single nucleotide polymorphisms (SNPs) that are associated with breast cancer, supporting the polygenic model (4). According to previous studies, these genetic variants that are associated with breast cancer, could be involved in different metabolisms and pathways, such as steroid hormone metabolism, detoxification of environmental carcinogens, tumor suppression, and DNA damage repair. Estrogen is one of the important factors in the carcinogenesis of breast cancer. It seems that this hormone is related with carcinogenesis of the mammary gland and progression of breast cancer. Therefore, studying genes that are related to biosynthesis and metabolism of estrogen could help identify possible candidate genes for breast cancer risk. One of these possible candidates is the CYP1A1 gene. CYP1A1 contributes to the metabolism of estrogens by converting estradiol to 2-hydroxyestradiol, the initial step in this pathway, and thus polymorphic variation in CYP1A1 activity could also affect breast cancer susceptibility by this mechanism. It has been proved that CYP1A1 metabolizes environmental carcinogens as well. Lipophilic aromatic hydrocarbons accumulate in mammary adipose tissue, and mammary epithelial cells are able to metabolize them to a reactive substance targeting DNA. Furthermore, CYP1A1 plays a role as an enzyme in phase I carcinogen metabolism by adding a hydroxyl group to carcinogenic compounds. Eventually, enzymes that are involved in Phase II, add an oxygen atom to the hydroxyl group, which turns these compounds into a water soluble derivative. The balance of activating enzymes (such as CYP1A1) to detoxifying enzymes governs the proportion of aromatic hydrocarbons that become DNA binding carcinogens (5). It is assumed that some genetic polymorphisms might modify the risk of breast cancer by influencing the CYP1A1 enzyme activity (6). Four single nucleotide polymorphisms in CYP1A1 have been identified, including T3801C, T3205C, A2455G (Ile462Val), and C2453A (Thr461Asp) (7). In the present work, the association between breast cancer and CYP1A1 Thr461Asn polymorphism was investigated. The function of this polymorphism has not been clearly established, although it has been suggested that it shows the greatest enzymatic efficiency amongst all CYP1A1 polymorphisms (8). The aim of this study was an evaluation of the C2453A (Thr461Asn) polymorphic form in CYP1A1 gene in an Iranian population.
2. Methods
2.1. Study Population
Breast cancer cases were selected by an oncologist from the Razi hospital (Iran). Females were asked to participate in the study and were interviewed before any procedure, and the study was approved by the oncologist. A total of 120 females were eventually diagnosed with confirmed breast cancer. Cases were females between the ages of 25 and 65 years from the Guilan province of Iran. Healthy population controls lived in the same area as the cases and had the same age range. Overall, 80 controls were chosen and interviewed in parallel with the cases; all of them agreed to participate in a genetic study.
2.2. Genotyping Analyses
Genomic DNA (100 ng) was extracted by the Cinnagen DNA extraction kit and used as a template in PCR-based Restriction Fragment Length Polymorphism (RFLP) assays. For the CYP1A1 Thr461Asn genotype determination, a 381-base pair (bp) fragment was amplified using the following primers (Generay Biotech):
CYP1A1(F)-5’CCCACAGCCCAGATAGCAA
CYP1A1(R)-5’CCCTGATGGTGCTATCGACA
The PCR amplification reactions were performed in 20-µL aliquots using 5 µL of genomic DNA, 1 µL of each primer, 3 µL of water, and 10 µL of PCR Master Mix (Cinnagen). Amplification was carried out for 35 cycles with the initial denaturation step at 95°C for 5 minutes and the following steps were repeated 35 times: denaturation at 95°C for 30 seconds, annealing at 59°C for 45 seconds, extension at 72°C for 1 minute, and a final extension at 72°C for 10 minutes. The amplified PCR products were digested with a specific restriction enzyme at 37°C for 2 hours and visualized using agarose gel electrophoresis. The restriction enzyme for the CYP1A1 codon 2453 genotype was BsmAI (Fermentas). Eventually, the chi-square test was used to compare the allele and genotype frequencies.
3. Results
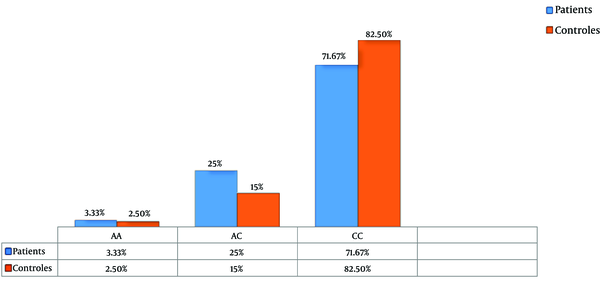
The product of amplification was digested with the BsmA1 restriction enzyme. After analysis of fragments with 1.5% agarose gel electrophoresis (CC homozygotes genotype with no mutation: two 306 bp and 75 bp fragments, AA mutant homozygotes: uncut 381 bp fragment, AC heterozygotes: 381 bp, 306 bp, and 75 bp three fragments) and calculating of genotype and allele frequencies by chi-squared test, genotype distributions of the CYP1A1 gene showed no significant difference between patients and controls (P = 0.2, Allele A in patients 0.16, in controls 0.1, Allele C in patients 0.84, and controls 0.9, respectively) (Table 1 and Figure 1). The results from this study suggest that the Thr461Asn polymorphism of CYP1A1 gene may not be associated with the risk of breast cancer.
Chi-Square Results for the CYP1A1 Gene Thr461Asn Polymorphism
| Alleles Genotypes | Controls N | Patients N | χ² | P Value |
|---|---|---|---|---|
| Genotypes | 3.13 | 0.2 | ||
| AA | 2 (2.5) | 4 (3.33) | ||
| AC | 12 (15) | 30 (25) | ||
| CC | 66 (82.5) | 86 (71.67) | ||
| Allels | 2.32 | 0.12 | ||
| A | 16 (0.1) | 38 (0.16) | ||
| C | 144 (0.9) | 202 (0.84) |
The Percentage of Genotype Frequencies in Patients and Control Cases
4. Discussion
In the recent decades, breast cancer has become one the major causes of death among females. Breast cancer is caused by environmental and genetic factors. Lifetime estrogen exposure is one of the risk factors that plays an important role in breast cancer. It seems that this hormone could influence the development of the mammary gland and breast cancer progression (9).
During each month of the menstrual cycle, in the uterus, estrogen causes the proliferation of endometrial cells, followed by death of these cells during menstruation. Similarly, during each menstrual cycle, estrogen normally triggers the proliferation of cells in the inner lining of the ducts in the breast. CYP1A1 is one the genes that is involved in the metabolism of estrogens. Several single nucleotide polymorphisms in CYP1A1 have been identified, such as T3801C, T3205C, A2455G (Ile462Val), and C2453A (Thr461Asp); and some of these polymorphisms seem to be associated with the risk of breast cancer development. In the present work, the researchers investigated the association between Thr461Asn polymorphism of CYP1A1 gene and breast cancer. Commonly, the results from different researches that worked on the relationship between the CYP1A1 polymorphism and breast cancer are conflicting. Some studies have shown an association with cancer in the presence of polymorphism, whereas others have reported no relationship. For example in 2013, Li investigated the association of the CYP1A1 gene Thr461Asn polymorphism with endometrial cancer and he reported that there was no relationship between this polymorphism and endometrial cancer (10). In 2012, Sergentanis analyzed the relationship of this polymorphism of CYP1A1 with ovarian cancer and he informed no association (11), and in 2001 in a research by Basham, after analysis of 1873 patients with breast cancer and 712 control cases, similar to the current study, he reported that there was no association between Thr461Asn polymorphism of CYP1A1 and breast cancer (5). In this research, genotype distributions of the CYP1A1 gene showed no significant difference between patients and controls (P = 0.2, Allele A in patients 0.16, in controls 0.1, Allele C in patients 0.84, and controls 0.9, respectively).
4.1. Conclusions
In conclusion, the results of this study suggest that the Thr461Asn polymorphism of CYP1A1 gene may not be associated with the risk of breast cancer in the Guilan province population of Iran. Further studies are needed to confirm the role of the CYP1A1 gene in breast cancer.
Acknowledgements
References
-
1.
Li W, Liu KJ, Song JS, Song R, Liu ZL. Association between RAD51 polymorphism and breast cancer susceptibility: a meta analysis. Int J Clin Exp Med. 2015;8(2):2326-33. [PubMed ID: 25932169].
-
2.
Rodrigues MS, Machado CA, Pagnoncelli D, Avvad E, Paixao JC, Gallo CV. TP53 and XRCC1 polymorphisms and breast cancer prognosis: a case-case study. Clinics (Sao Paulo). 2011;66(6):1097-100. [PubMed ID: 21808882].
-
3.
Lose F, Lovelock P, Chenevix-Trench G, Mann GJ, Pupo GM, Spurdle AB, et al. Variation in the RAD51 gene and familial breast cancer. Breast Cancer Res. 2006;8(3):R26. [PubMed ID: 16762046]. https://doi.org/10.1186/bcr1415.
-
4.
Sapkota Y, Mackey JR, Lai R, Franco-Villalobos C, Lupichuk S, Robson PJ, et al. Assessing SNP-SNP interactions among DNA repair, modification and metabolism related pathway genes in breast cancer susceptibility. PLoS One. 2014;8(6). e64896. [PubMed ID: 23755158]. https://doi.org/10.1371/journal.pone.0064896.
-
5.
Basham VM, Pharoah PD, Healey CS, Luben RN, Day NE, Easton DF, et al. Polymorphisms in CYP1A1 and smoking: no association with breast cancer risk. Carcinogenesis. 2001;22(11):1797-800. [PubMed ID: 11698341].
-
6.
Miyoshi Y, Takahashi Y, Egawa C, Noguchi S. Breast cancer risk associated with CYP1A1 genetic polymorphisms in Japanese women. Breast J. 2002;8(4):209-15. [PubMed ID: 12100112].
-
7.
Sergentanis TN, Economopoulos KP. Four polymorphisms in cytochrome P450 1A1 (CYP1A1) gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;122(2):459-69. [PubMed ID: 20035380]. https://doi.org/10.1007/s10549-009-0694-5.
-
8.
Wright CM, Larsen JE, Colosimo ML, Barr JJ, Chen L, McLachlan RE, et al. Genetic association study of CYP1A1 polymorphisms identifies risk haplotypes in nonsmall cell lung cancer. Eur Respir J. 2010;35(1):152-9. [PubMed ID: 19608585]. https://doi.org/10.1183/09031936.00120808.
-
9.
Germain D. Estrogen carcinogenesis in breast cancer. Endocrinol Metab Clin North Am. 2011;40(3):473-84. vii. [PubMed ID: 21889715]. https://doi.org/10.1016/j.ecl.2011.05.009.
-
10.
Li M, Li YY, Xin XY, Han Y, Wu TT, Wang HB. Quantitative assessment of the association between CYP1A1 A4889G polymorphism and endometrial cancer risk. Tumour Biol. 2013;34(6):3675-80. [PubMed ID: 23860774]. https://doi.org/10.1007/s13277-013-0949-y.
-
11.
Sergentanis TN, Economopoulos KP, Choussein S, Vlahos NF. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and ovarian cancer risk: a meta-analysis. Mol Biol Rep. 2012;39(11):9921-30. [PubMed ID: 22733497]. https://doi.org/10.1007/s11033-012-1860-0.