Abstract
Background:
Atorvastatin exerts neuroprotective effects on the treatment of central nervous system disorders. Morphine analgesic tolerance and dependence remain as major concerns in medicine. Nitric oxide (NO) pathway mediates the development of opioid analgesic tolerance and dependence, as well as atorvastatin neuroprotection.Objectives:
The present study aimed to assess the possible involvement of the NO/cGMP pathway in the process of the effects of atorvastatin on morphine physical dependence.Methods:
Dependence was induced by repetitive injection of morphine sulfate. Naloxone was injected at the dose of 4 mg/kg on the last day of the experiment to assess withdrawal signs. Animals received atorvastatin (1, 5, 10, and 20 mg/kg, orally). Nitric oxide synthase (NOS) inhibitors and ODQ were injected before protective dose of atorvastatin. The gene expression of NOS isoforms was evaluated by real-time PCR. Thereafter, the hippocampal levels of cGMP and nitrite were measured.Results:
Treatment with atorvastatin 10 mg/kg significantly attenuated naloxone-induced withdrawal behaviours. The administration of L-NAME, aminoguanidine, and ODQ before atorvastatin enhanced its effects. The treatment with atorvastatin significantly decreased the nitrite and cGMP levels as well as NOS gene expression in the hippocampus of dependent animals.Conclusions:
It can be concluded that atorvastatin, possibly, through inducible NOS, could alleviate morphine dependence and withdrawal signs.Keywords
1. Background
Atorvastatin, as a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, is a well-tolerated drug commonly known for its effect on improving the lipid profile. Previous studies have implicated that atorvastatin has numerous protective effects under different pathological conditions in the central nervous system (CNS) (1, 2).
Investigations have been conducted on the effect of statins on morphine analgesic tolerance or dependence (3). Accordingly, one study reported that acute and chronic administration of simvastatin could alleviate morphine-induced both analgesic tolerance and dependence (4). In our previous study, we have found that oral administration of atorvastatin could attenuate antinociceptive tolerance induced via morphine’s repetitive injection to mice (5). Pajohanfar et al. (6) in their study showed the protection of atorvastatin against morphine analgesic tolerance and behavioural changes of withdrawal. Li et al. (3) have also identified an attenuating property of rosuvastatin in morphine analgesic tolerance.
One of the main limiting factors for the long-term administration of morphine or other opioids is the risk of the development of dependence (7). Morphine-induced dependence consists of two components as follows: the first one is physical dependence defined as expressing withdrawal signs following drug cessation or antagonist administration, and the other one is psychological dependence known as a mental state for obsessive and continuous seeking for drug consumption (8). A large body of evidence has demonstrated different mechanisms for the development of opioids dependence including glial cell activation, activation of protein kinase C (PKC), desensitization of mu-opioid receptor, and the increased nitric oxide production (8-10).
Nitric oxide (NO) is known as a potent guanylyl cyclase stimulator and also as an important neurotransmitter that mediates numerous neurological processes (11). It is well-established that NO pathway plays a crucial role in developing morphine-derived physical dependence. A study by Abdel-Zaher et al. (10) showed that blocking the NO overproduction via the administration of aminoguanidine, which is an inducible NOS (iNOS) inhibitor, could alleviate the development of morphine dependence. In addition, Kolesnikov et al. (12, 13) showed the role of NO in morphine tolerance and dependence. Moreover, NO/cGMP has an important contribution to the beneficial effects of atorvastatin on CNS (14). Shafaroodi et al. (1) in their study demonstrated the involvement of NO in anti-seizure properties of acute atorvastatin. Another study concluded that NO has an effect on spatial memory improvement via atorvastatin (15). Furthermore, statins are considered as novel modulators in CNS processes through different mechanisms including eNOS or nNOS regulation (14).
2. Objectives
This study aimed to investigate the effect of atorvastatin on physical dependence to morphine in mice as well as the involvement of NO/cGMP pathway in the modulating effects of atorvastatin.
3. Methods
3.1. Animals
Male white Albino NMRI mice (Tehran University of Medical Sciences, Iran), weighing 25 ± 4 g were used in this experimental study. The animals were kept in cages (4-6 mice) under the standard laboratory conditions in a 12-hour light/dark cycle at 22 ± 2°C and humidity 55 ± 2%. Additionally, they had free access to food and water. Notably, each mouse was used only once during the study. These animals were cared and treated in terms of the institutional Guideline for the Care and Use of Laboratory Animals (NIH publication no.: 86-23, revised 1996) and approval of the Institutional Animal Care and Use Committee (IACUC) of Tehran University of Medical Sciences, Tehran, Iran.
3.2. Chemicals
The drugs used throughout the study were as follows: Atorvastatin, a HMG-CoA inhibitor (Sobhan Darou Co., Tehran, Iran); morphine sulfate, an opioid agonist (Temad, Iran); naloxone hydrochloride, a non-selective opioid receptor antagonist (Tolid Daru Co., Tehran, Iran); L-NAME, a non-specific inhibitor of NOS (Sigma, USA); aminoguanidine, a selective inhibitor of inducible NOS (iNOS) (Sigma, USA); 7-nitroindazole, a selective inhibitor of neuronal NOS (nNOS) (Sigma, USA); and ODQ, a selective and potent soluble guanylyl cyclase (sGC) inhibitor (Sigma, USA). All these drugs were administered by intraperitoneal injection (i.p.) in a volume of 10 mL/kg of the mice body weight, except for atorvastatin suspension that was prepared in carboxymethyl cellulose (CMC, 0.5%) and then administered by oral gavage. Morphine, L-NAME, and aminoguanidine were dissolved in sterile isotonic saline solution. In addition, 7-nitroindazole and ODQ were prepared in sterile isotonic saline using DMSO 4% as the co-solvent.
3.3. Induction of Physical Dependence of Morphine
Physical dependence was induced by the repeated injection of morphine. Morphine sulfate was injected three times per day with doses of 50 mg/kg at 8:00 A.M., 50 mg/kg at 11:00 A.M., and 75 mg/kg at 4:00 P.M.; the last dose was to prevent withdrawal signs overnight. On day 5 of the experiment, withdrawal behaviours were induced by the administration of naloxone (4 mg/kg, i.p.) by passing one hour from the injection of morphine sulfate (100 mg/kg) at 9:00 A.M. Immediately after naloxone injection, each mouse was separately placed in a plexiglass box (45 cm high, 40 cm long, and 25 cm wide) and was then followed-up for 45 minutes to monitor naloxone-induced withdrawal symptoms, including number of jumps, rearing, and initiation of grooming. Weight loss for each one of the animals was determined by comparing animal weights once before the naloxone administration and once at the end of the 45-minute observation period (5, 16, 17). So the group one is as follow:
Group 1: Morphine dissolved in saline and administered three times daily and assessed for naloxone test (n = 8).
3.4. Effects of Atorvastatin Treatment on Morphine-Dependence as Well as Determining the Role of NO
To assess the atorvastatin chronic effects on dependence, four different doses of atorvastatin (1 mg/kg, 5 mg/kg, 10 mg/kg, and 20 mg/kg) were orally administered 45 min before first dose of morphine once daily for 4 consecutive days.
Different experimental groups in this section were as follows (n = 8):
Group 2: CMC as vehicle suspended in saline and administered once daily.
Group 3-6: Atorvastatin with the doses of 1, 5, 10, and 20 mg/kg simultaneously with the first dose of morphine (once daily).
These doses of atorvastatin had no effect on withdrawal signs per se (doses selected based on previous studies).
In order to determine the contribution of NO pathway to the effects of atorvastatin on physical dependence, NOS inhibitors, including 7-nitroindazole (15 mg/kg), L-NAME with the dose of 2 mg/kg, aminoguanidine with the dose of 50 mg/kg, and ODQ (10 mg/kg) were administrated 30 min before atorvastatin (75 min before the administration of morphine) once per day for 4 consecutive days (5). The groups are as follow (n = 8).
Group 7: Atorvastatin effective dose (obtained from previous experiment) with L-NAME under the morphine treatment (MOR + ATOR10 + LNM).
Group 8: Atorvastatin effective dose (obtained from previous experiment) with aminoguanidine under the morphine treatment (MOR + ATOR10 + AG).
Group 9: Atorvastatin effective dose (obtained from previous experiment) with 7-nitroindazole under the morphine treatment (MOR + ATOR10 + 7NI).
Group 10: Atorvastatin effective dose (obtained from previous experiment) with ODQ under the morphine treatment (MOR + ATOR10 + ODQ).
3.5. Measurement of mRNA Expression of NOS Hippocampus’s Enzymes via Real-time PCR
The following groups were selected for performing this analysis: (1) vehicle group, (2) morphine-dependent group, (3) atorvastatin 10 mg/kg treated group, with no morphine dependence, and (4) morphine-dependent animals treated with atorvastatin 10 mg/kg. Some animals from section 3-3 and 3-4 were selected for this assessment. Animals in the above-mentioned groups were sacrificed by cervical dislocation under mild anesthesia via diethyl ether immediately after termination of the experiments. Hippocampus was dissected from the entire anterior to posterior brain axis on ice-cold surface, immediately snapped freeze in the liquid nitrogen, and finally maintained at -80°C. The prepared tissues were homogenized and then total RNA was extracted using RNA easy mini kit (Qiagen, USA) in terms of the manufacturer’s instructions. Subsequently, single strand cDNA was obtained through cDNA kit followed by the amplification of the specific mRNA. The changes in mRNA expression of inducible NOS, neuronal NOS, and endothelial NOS were measured by qRT-PCR on a StepOne Plus instrument (Applied Biosystems) using qRT-PCR Master Mix kit (Ampliqon, Copenhagen, Denmark).
The primers sequences (Takapouzist Co. Iran) used for the PCR amplification were as follows: iNOS (forward: GTTCTCAGCCCAACAATACAAGA; reverse: GTGGACGGGTCGATGTCAC); nNOS (forward: AATGGGTCTTGTGTATGCTAGG; reverse: ATGAAGATGGGAAGGAGTTGG); eNOS (forward: AACCATTCTGTATGGCTCTGAGAC; reverse: CTCTAGGGACACCACATCATACTC); β-Actin: forward: GTGACGTTGACATCCGTAAAGA; reverse: GCCGGACTCATCGTACTCC. The expression of mRNA level was normalized to β-Actin in each reaction. Notably, the step of activation was 15 min at 95°C, which was followed by 40 cycles including the denaturation step for 10 min at 95°C and annealing/extension step for 1 min at 60°C. Moreover, for calculation, 2-ΔΔCT formula was used (16, 18).
3.6. Nitrite Assay in the Hippocampus
This assessment was performed on the following groups: (1) Vehicle group; (2) morphine-dependent group; (3) atorvastatin 10 mg/kg treated group, with no morphine dependence; and (4) morphine-dependent animals treated with atorvastatin 10 mg/kg. Of note, separate groups of animals were used for this assay (n = 4/group). The hippocampus was isolated based on the approach described in Section 3.5. Nitrite level evaluation was performed by Griess reaction method. The obtained tissues (approximately 80 mg) were homogenized via lysis buffer solution. Additionally, the homogenized samples were centrifuged for 15 min at 3400 g (4°C). The supernatants obtained from the centrifugation were pipetted into a 96-well microtiter plate. Afterward, a volume of Griess reagent (0.1 mL) including sulphanilamide and N-(1-naphtyl) ethylenediamine hydrochloride, was added and then allowed to incubate for 15 min at room temperature to develop colour. Chromophore azo-derivative absorption was read at 540 nm using a microplate ELISA reader. The concentration of nitrite was measured through comparing the absorption of our samples with that of sodium nitrite standard solutions’ absorption (19).
3.7. Determination of cGMP Level in Hippocampus
This level was determined on the following groups: (1) Control group; (2) morphine-dependent group; (3) atorvastatin 10 mg/kg treated group with no morphine dependence; and (4) morphine-dependent animals treated with atorvastatin 10 mg/kg (n = 4 in each group). The hippocampus was isolated based on the approach described in section 3.5. The level of cGMP was measured using cGMP direct in vitro competitive ELISA kit (ab133027, abcam, USA). We prepared the frozen hippocampi, standards, and reagents in terms of the manufacturer’s instruction. Afterward, the test samples or standards along with alkaline phosphatase conjugated-cGMP antigen and polyclonal rabbit antibody specific to cGMP were added to the wells pre-coated with anti-rabbit IgG antibody. Following the incubation and washing away the excess reagents, pNpp substrate was added and after a short period of incubation, the enzymatic reaction was stopped. The generated yellow colour by this reaction was read at 405 nm. The results were reported as relative percentages compared to the control group.
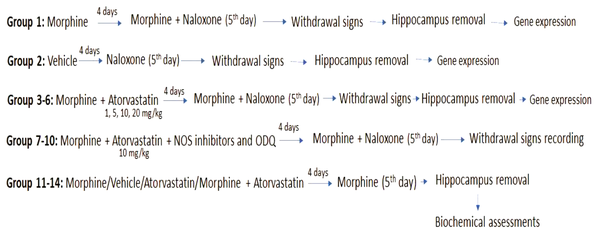
Figure 1 shows all the experimental groups in this study.
Schematic representation of drug administration for behavioural, gene expression and biochemical assessments.
3.8. Statistical Analysis
Data were analysed using GraphPad Prism data analysis program (version 6). One-way analysis of variance (ANOVA) was used for the treatment effects and post-hoc Tukey’s multiple comparisons were used to determine the differences among the experimental groups. The results are expressed as mean ± standard error of the mean (S.E.M). P < 0.05 was considered as statistically significance.
4. Results
4.1. The Effects of Chronic Atorvastatin Administration on Withdrawal Parameters
In order to show the effects of atorvastatin doses on the morphine dependence phenomenon, we co-treated atorvastatin with morphine. Our results are presented in Table 1. These data represented that morphine could increase the jumping number, rearing, grooming, and weight loss percentage compared to the vehicle-treated group (all P < 0.001). When we used atorvastatin with morphine chronically, it was observed that the dose of 10 mg/kg atorvastatin has a protection property against withdrawal manifestations. Weight loss percentage showed a reduction in comparison with the treatment by morphine alone (P < 0.05). Moreover, the number of jumping (P < 0.05), rearing (P < 0.01), and grooming (P < 0.05) showed a significant decrease after the administration of atorvastatin with the dose of 10 mg/kg. So, it can be stated that chronic atorvastatin treatment could attenuate the naloxone-precipitated withdrawal syndromes in physically dependent animals.
The Effects of Administration of Chronic Doses of Atorvastatin on the Withdrawal Manifestation Due to Naloxone in the Morphine Dependent Animals (N = 8 for Each Group)
| Treatments | Weight Change, % | Jumping | Rearing | Grooming |
|---|---|---|---|---|
| Vehicle | 6.3 ± 1.31 | 2.5 ± 0.64 | 11.40 ± 0.50 | 12.25 ± 0.85 |
| MOR | -6.1 ± 1.22*** | 23.8 ± 2.51*** | 33 ± 2.38*** | 42.25 ± 2.21*** |
| ATOR 1 + MOR | -4.85 ± 2.01 | 26.5 ± 2.39 | 36.4 ± 2.56 | 38.75 ± 4.09 |
| ATOR 5 + MOR | -6.75 ± 1.03 | 25.20 ± 3.9 | 35 ± 1.44 | 34.75 ± 3.06 |
| ATOR 10 + MOR | 1.57 ± 1.58# | 14.2 ± 1.53# | 25.4 ± 1.91## | 29 ± 2.48# |
| ATOR 20 + MOR | -6.15 ± 1.15 | 29 ± 3.24 | 39.8 ± 1.71 | 41 ± 3.02 |
4.2. The Effects of NOS Inhibitors and ODQ
Following the determination of the protective dose of atorvastatin (10 mg/kg), we have administered NOS inhibitors (non-specific: L-NAME; nNOS selective: 7-nitroindazole; and iNOS selective: aminoguanidine) and ODQ (as a highly selective and irreversible inhibitor of soluble guanylyl cyclase) simultaneously with atorvastatin plus morphine, to evaluate the effect of NO/cGMP pathway on the protective role of atorvastatin.
As shown in Table 2, L-NAME could decrease some signs of withdrawal such as jumping (P < 0.05), rearing (P < 0.01), and grooming (P < 0.05) in comparison with atorvastatin 10 mg/kg. In the present study, aminoguanidine reduced the number of grooming with P < 0.01. Of note, ODQ had diminishing effects on the two parameters of rearing and grooming (both P < 0.01). However, the administration of 7-nitroindazole with atorvastatin showed no significant changes in withdrawal sign in comparison to the atorvastatin treated group. These data indicated the involvement of nitrergic system in atorvastatin effects on naloxone-precipitated withdrawal signs.
The Effects of NOS Inhibitors and ODQ Administration on the Naloxone-Induced Withdrawal Manifestation in the Morphine Dependent Animals (N = 8 for Each Group)
| Treatments | Weight change, % | Jumping | Rearing | Grooming |
|---|---|---|---|---|
| MOR + ATOR10 + LNM | -0.9 ± 1.25 | 6.25 ± 1.54* | 11.4 ± 0.5** | 19.5 ± 0.95* |
| MOR + ATOR10 + AG | -4.23 ± 1.08 | 8 ± 1.29 | 18 ± 1.08 | 17 ± 0.89** |
| MOR + ATOR10 + 7NI | -2.56 ± 2.45 | 15.25 ± 1.49 | 22 ± 2.56 | 22 ± 2.61 |
| MOR + ATOR10 + ODQ | -2.07 ± 1.57 | 6.75 ± 1.03 | 11.5 ± 1.04** | 16.5 ± 2.1** |
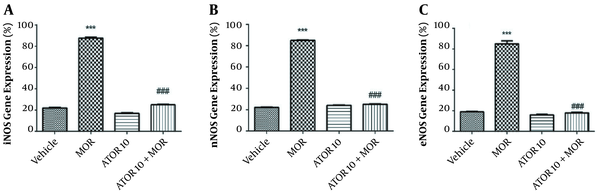
4.3. mRNA Expression Results
The effects of atorvastatin on the mRNA expression of NOS isoforms are shown in Figure 2. In the current study, we have isolated the hippocampus of mice from the following groups: (1) groups chronically treated with atorvastatin 10 mg/kg alone and in combination with morphine; (2) vehicle group; and (3) morphine dependent group. Thereafter, we evaluated the mRNA expressions of eNOS, iNOS, and nNOS in order to confirm the involvement of NO in atorvastatin effects. These data showed that morphine increases the relative gene expression of iNOS when compared with the control animals (P < 0.001); however, the administration of atorvastatin with morphine could significantly reverse this enhancement [depicted in Figure 2, part A]. The expression of nNOS mRNA by RT-PCR showed that morphine dependence leads to a significant increase (P < 0.001), but when co-administered with atorvastatin, this effect would be significantly blocked (P < 0.001) [Figure 2, part B]. These patterns of expression were also repeated for eNOS as pictured in part C of Figure 2; morphine injection enhanced the eNOS expression in animals’ hippocampus, but atorvastatin at the dose of 10 mg/kg prevented this effect. These data led us to gain better understanding on the NO involvement in atorvastatin’s function on morphine dependence.
NOS mRNA expression in the hippocampus of morphine (MOR)-dependent mice. Animals received MOR for 5 days. Atorvastatin (ATOR) was administered with the dose of 10 mg/kg (chronic) alone or 45 min before MOR. Data are expressed as mean ± S.E.M. ***, P < 0.001 compared to vehicle; ###, P < 0.001 compared to the MOR-treated animals.
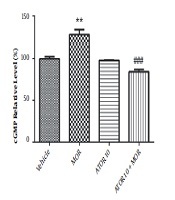
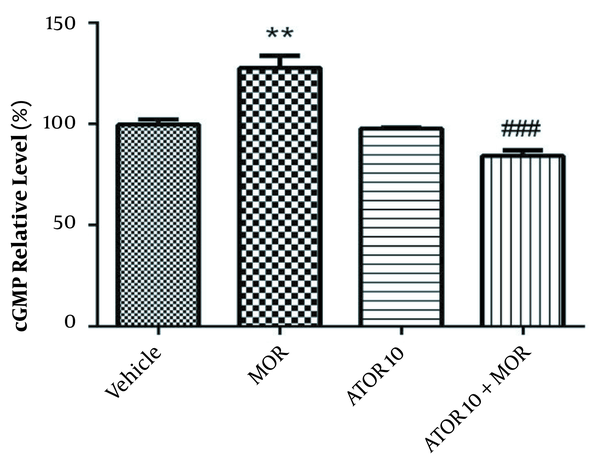
4.4. The Hippocampus cGMP Level Results
The cGMP level was measured using enzyme-linked immunosorbent assay in the group of vehicle, morphine group, atorvastatin 10 mg/kg alone, and simultaneously administered with morphine (as described previously in material and methods section). Guanylyl cyclase catalyses the synthesis of cGMP from GTP and soluble guanylyl cyclase is activated via NO (20). Regarding Figure 3, our data showed that morphine treatment results in the increased cGMP relative percentage (P < 0.01). Atorvastatin (P < 0.001) treatment with morphine was also accompanied by lowering the morphine-induced enhancement of cGMP percentage.
The relative percentage of cGMP in hippocampus of morphine (MOR)-dependent mice. Animals received MOR for 5 days. Atorvastatin (ATOR) was administered with the dose of 10 mg/kg alone or in combination with MOR (45 min before MOR). Data are represented as mean ± S.E.M (N = 6 in every treatment group). **, P < 0.01 compared to vehicle; ###, P < 0.001compared to the MOR-treated animals.
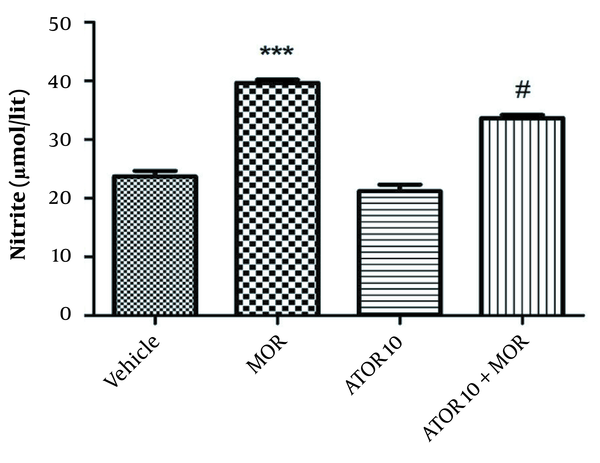
4.5. The Effects of Atorvastatin Treatment on Nitrite Level in the Dependent Animals
As indicated in Figure 4, our data showed a rise in nitrite level in the group dependent to morphine in comparison with the vehicle animals (P < 0.001). Using atorvastatin with morphine resulted in a decline in hippocampus nitrite level (P < 0.05) when compared with the morphine dependent animals.
The hippocampus level of nitrite in the morphine (MOR)-dependent mice. Animals received MOR for 5 days. Atorvastatin (ATOR) was administered alone and 45 min before MOR. Data are expressed as mean ± S.E.M (N = 6 in every treatment group). ***, P < 0.001 compared to the vehicle; #, P < 0.05 compared to MOR-treated animals.
5. Discussion
This study exhibited that administration of atorvastatin could significantly reduce withdrawal signs. In addition, in this research, the treatment with NOS inhibitors (L-NAME and aminoguanidine) or guanylyl cyclase inhibitor (ODQ) led to the escalation of atorvastatin effects on some parameters such as in rearing and grooming. The chronic administration of atorvastatin was found to be associated with the reduction of morphine-induced rise of inducible NOS expression as well as eNOS and nNOS expressions. The decreased cGMP level was observed in the morphine plus atorvastatin group, while it was elevated in the morphine-treated animals. Nitrite, as an end product of NO, displayed a reduction with the chronic atorvastatin administration in the morphine dependent mice.
Atorvastatin has gained attention for its neuroprotective effects on different CNS disorders (1). Recently, statins were explored as interesting targets for preventing morphine analgesic tolerance and physical dependence. Hassanipour et al. (5) investigated the role of atorvastatin in analgesic tolerance and then reported that atorvastatin blocks tolerance in dependent mice. Notably, simvastatin blocked the tolerance and dependence to morphine in one experimental study (4). There is an evidence of delaying and partially reversing the morphine analgesic tolerance in rats treated by rosuvastatin (21). Moreover, Pajohanfar et al. (6) demonstrated that chronic treatment with atorvastatin for nine days would protect animals against tolerance and dependence to morphine through the inhibition of glia activity or antioxidant effect. Moreover, opioids mediate some beneficial effects of statins. In this regard, Dolatshahi et al. (22) showed that opioid pathways are involved in the antidepressant-like effect of simvastatin without any induction of tolerance or withdrawal sign. Our study paralleled to other studies supports the hypothesis that statins may be beneficial in the challenges resulted from morphine use. This study showed that the chronic administration of atorvastatin reduces the withdrawal signs.
Nitric oxide (NO) is synthesized from L-arginine via NOS enzymes (iNOS, eNOS, and nNOS) (1). Different studies indicated that NO-NOS/GC (guanylyl cyclase)/cGMP (cyclic guanosine mono phosphate) has contribution into the development of opioids analgesic tolerance and physical dependence (23, 24). It was demonstrated that the repeated morphine treatment causes an increase in the secretion of some mediators including NO (17). Inhibition of NOS and the prevention of NO overproduction diminished morphine tolerance (25). Studies provided evidence that blockade of NO with aminoguanidine could decrease the development of morphine tolerance and dependence through the inhibition of iNOS, revealing the involvement of iNOS in these phenomena (10, 26). The data obtained in our study also indicated the involvement of NO pathway as a mechanism for the effect of atorvastatin on morphine physical dependence.
Many studies demonstrated that a variety of cholesterol-independent pleiotropic effects of statins could be mediated through NO/cGMP pathway (27). Accordingly, Moezi et al. (20) showed that NO signalling could mediate the anticonvulsant effect of atorvastatin probably through iNOS. In a study it was revealed that rosuvastatin could improve the vascular structure or function via enhancing the eNOS expression (28). In our previous study, we have shown that improving the effects of atorvastatin on morphine analgesic tolerance may be regulated by NOS inhibitors (5). In this study, treatment with iNOS inhibitor in the dependent mice intensified the atorvastatin property; showing that atorvastatin exerts its protective function possibly via NO reduction. Moreover, in our study, gene expression of NOSs and nitrite and cGMP level measurement confirmed the role of nitrergic pathway in atorvastatin effects. To describe it more, NOS inhibitors (selective and non-selective) were co-administered with atorvastatin and morphine and these data showed that atorvastatin effect was improved. We have hypothesised that atorvastatin may prevent naloxone-induced withdrawals by nitric oxide production’s blockade and an additive effect has occurred when the NOS inhibitors were co-administered with atorvastatin. So, in some behavioural parameters, we have observed an additional improvement in withdrawal signs even more than atorvastatin alone. Correspondingly, this improvement has been observed with L-NAME and aminoguanidine; showing that iNOS is involved and atorvastatin could act by iNOS inhibition. In our study, the improvement in withdrawal behaviours was not observed with nNOS inhibitor. But, nNOS plays a role in morphine dependence (29), and it can be modulated through statins such as atorvastatin (30). Besides, our gene expression results showed that atorvastatin affect the level of nNOS and even eNOS in hippocampus and this can be considered as the reason for better action of the L-NAME group (morphine + atorvastatin 10 mg/kg + L-NAME) compared to the aminoguanidine group (morphine + atorvastatin 10 mg/kg + aminoguanidine) in reducing withdrawal signs.
As morphine analgesic tolerance and dependence are important challenges in clinical setting, finding new approaches to impede these phenomena seems necessary and statins may be relatively known as safe adjuvant therapies. However, more investigations are needed to elucidate the exact mechanisms and neural signalling in various brain areas involved in morphine tolerance and dependence. So, future studies should be performed on some subjects such as protein expression of iNOS and nNOS, determining the role of PKC (data showed that some statin effects could be mediated via PKC pathway (31)), evaluating NO/cGMP in other areas of the brain and role of inflammation.
5.1. Conclusions
In conclusion, the results of the present study show that atorvastatin could mitigate withdrawal signs induced by naloxone. Atorvastatin effects were improved by non-selective NOS inhibitor, iNOS and guanylyl cyclase inhibitors. In addition, the expression of NOS isoforms has decreased after the treatment by atorvastatin. The levels of nitrite and cGMP have also reduced by atorvastatin in the hippocampus of the morphine-dependent mice. We, therefore, suggest that chronic atorvastatin effects on morphine-dependence may be mediated via iNOS and cGMP.
References
-
1.
Shafaroodi H, Moezi L, Fakhrzad A, Hassanipour M, Rezayat M, Dehpour AR. The involvement of nitric oxide in the anti-seizure effect of acute atorvastatin treatment in mice. Neurol Res. 2012;34(9):847-53. [PubMed ID: 22909758]. https://doi.org/10.1179/1743132812Y.0000000080.
-
2.
Kumar A, Sharma N, Gupta A, Kalonia H, Mishra J. Neuroprotective potential of atorvastatin and simvastatin (HMG-CoA reductase inhibitors) against 6-hydroxydopamine (6-OHDA) induced Parkinson-like symptoms. Brain Res. 2012;1471:13-22. [PubMed ID: 22789904]. https://doi.org/10.1016/j.brainres.2012.06.050.
-
3.
Li W, Li Y, Zhu S, Ji Q, Shu Y, Zhang L, et al. Rosuvastatin attenuated the existing morphine tolerance in rats with L5 spinal nerve transection through inhibiting activation of astrocytes and phosphorylation of ERK42/44. Neurosci Lett. 2015;584:314-9. [PubMed ID: 25445360]. https://doi.org/10.1016/j.neulet.2014.11.003.
-
4.
Mansouri MT, Khodayar MJ, Tabatabaee A, Ghorbanzadeh B, Naghizadeh B. Modulation of morphine antinociceptive tolerance and physical dependence by co-administration of simvastatin. Pharmacol Biochem Behav. 2015;137:38-43. [PubMed ID: 26255154]. https://doi.org/10.1016/j.pbb.2015.08.002.
-
5.
Hassanipour M, Amini-Khoei H, Shafaroodi H, Shirzadian A, Rahimi N, Imran-Khan M, et al. Atorvastatin attenuates the antinociceptive tolerance of morphine via nitric oxide dependent pathway in male mice. Brain Res Bull. 2016;125:173-80. [PubMed ID: 27381980]. https://doi.org/10.1016/j.brainresbull.2016.07.002.
-
6.
Pajohanfar NS, Mohebbi E, Rad A, Pejhan A, Nazemi S, Amin B. Protective effects of atorvastatin against morphine-induced tolerance and dependence in mice. Brain Res. 2017;1657:333-9. [PubMed ID: 28062186]. https://doi.org/10.1016/j.brainres.2016.12.028.
-
7.
Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164(4):1322-34. [PubMed ID: 21434879]. [PubMed Central ID: PMC3229764]. https://doi.org/10.1111/j.1476-5381.2011.01335.x.
-
8.
Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5(1):60-8. [PubMed ID: 15661627]. https://doi.org/10.1016/j.coph.2004.08.012.
-
9.
Mayer DJ, Mao J, Price DD. The development of morphine tolerance and dependence is associated with translocation of protein kinase C. Pain. 1995;61(3):365-74. [PubMed ID: 7478679]. https://doi.org/10.1016/0304-3959(95)00023-L.
-
10.
Abdel-Zaher AO, Hamdy MM, Aly SA, Abdel-Hady RH, Abdel-Rahman S. Attenuation of morphine tolerance and dependence by aminoguanidine in mice. Eur J Pharmacol. 2006;540(1-3):60-6. [PubMed ID: 16730698]. https://doi.org/10.1016/j.ejphar.2006.03.059.
-
11.
Chabrier PE, Demerle-Pallardy C, Auguet M. Nitric oxide synthases: targets for therapeutic strategies in neurological diseases. Cell Mol Life Sci. 1999;55(8-9):1029-35. [PubMed ID: 10442086]. https://doi.org/10.1007/s000180050353.
-
12.
Kolesnikov YA, Pick CG, Pasternak GW. NG-nitro-L-arginine prevents morphine tolerance. Eur J Pharmacol. 1992;221(2-3):399-400. [PubMed ID: 1426018]. https://doi.org/10.1016/0014-2999(92)90732-j.
-
13.
Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc Natl Acad Sci U S A. 1993;90(11):5162-6. [PubMed ID: 7685116]. [PubMed Central ID: PMC46675]. https://doi.org/10.1073/pnas.90.11.5162.
-
14.
Fracassi A, Marangoni M, Rosso P, Pallottini V, Fioramonti M, Siteni S, et al. Statins and the Brain: More than Lipid Lowering Agents? Curr Neuropharmacol. 2019;17(1):59-83. [PubMed ID: 28676012]. [PubMed Central ID: PMC6341496]. https://doi.org/10.2174/1570159X15666170703101816.
-
15.
Javadi-Paydar M, Rayatnia F, Fakhraei N, Zakeri M, Mirazi N, Norouzi A, et al. Atorvastatin improved scopolamine-induced impairment in memory acquisition in mice: involvement of nitric oxide. Brain Res. 2011;1386:89-99. [PubMed ID: 21354117]. https://doi.org/10.1016/j.brainres.2011.02.057.
-
16.
Javadi S, Ejtemaeimehr S, Keyvanfar HR, Moghaddas P, Aminian A, Rajabzadeh A, et al. Pioglitazone potentiates development of morphine-dependence in mice: possible role of NO/cGMP pathway. Brain Res. 2013;1510:22-37. [PubMed ID: 23399681]. https://doi.org/10.1016/j.brainres.2012.12.035.
-
17.
Mansouri MT, Naghizadeh B, Ghorbanzadeh B, Amirgholami N, Houshmand G, Alboghobeish S. Venlafaxine inhibits naloxone-precipitated morphine withdrawal symptoms: Role of inflammatory cytokines and nitric oxide. Metab Brain Dis. 2020;35(2):305-13. [PubMed ID: 31630319]. https://doi.org/10.1007/s11011-019-00491-4.
-
18.
Mumtaz F, Shafaroodi H, Nezamoleslami S, Zubair M, Sheibani M, Nikoui V, et al. Involvement of nNOS, and alpha1, alpha2, beta1, and beta2 Subunits of Soluble Guanylyl Cyclase Genes Expression in Anticonvulsant Effect of Sumatriptan on Pentylenetetrazole-Induced Seizure in Mice. Iran J Pharm Res. 2020;19(4):181-92. [PubMed ID: 33841534]. [PubMed Central ID: PMC8019868]. https://doi.org/10.22037/ijpr.2020.112594.13844.
-
19.
Rahimi N, Dehpour AR, Javadi-Paydar M, Sohanaki H, Rabbani S, Ansari M, et al. Effect of DETA-NONOate and papaverine on vasodilation of human internal mammary artery. Can J Physiol Pharmacol. 2011;89(12):945-51. [PubMed ID: 22117187]. https://doi.org/10.1139/y11-092.
-
20.
Moezi L, Shafaroodi H, Hassanipour M, Fakhrzad A, Hassanpour S, Dehpour AR. Chronic administration of atorvastatin induced anti-convulsant effects in mice: the role of nitric oxide. Epilepsy Behav. 2012;23(4):399-404. [PubMed ID: 22405864]. https://doi.org/10.1016/j.yebeh.2012.02.001.
-
21.
Li Y, Shu Y, Ji Q, Liu J, He X, Li W. Attenuation of morphine analgesic tolerance by rosuvastatin in naive and morphine tolerance rats. Inflammation. 2015;38(1):134-41. [PubMed ID: 25261133]. https://doi.org/10.1007/s10753-014-0015-y.
-
22.
Dolatshahi M, Davoudi S, Paridar Y, Naserzadeh R, Ghorbanzadeh B. Pharmacological evidence for the involvement of the opioid system in the antidepressant-like effect of simvastatin in mice: Without tolerance and withdrawal syndrome. Neurosci Lett. 2020;714:134578. [PubMed ID: 31669314]. https://doi.org/10.1016/j.neulet.2019.134578.
-
23.
Zamanian G, Shayan M, Rahimi N, Bahremand T, Shafaroodi H, Ejtemaei-Mehr S, et al. Interaction of morphine tolerance with pentylenetetrazole-induced seizure threshold in mice: The role of NMDA-receptor/NO pathway. Epilepsy Behav. 2020;112:107343. [PubMed ID: 32755816]. https://doi.org/10.1016/j.yebeh.2020.107343.
-
24.
Wolinska R, Kleczkowska P, de Corde-Skurska A, Poznanski P, Sacharczuk M, Mika J, et al. Nitric oxide modulates tapentadol antinociceptive tolerance and physical dependence. Eur J Pharmacol. 2021;907:174245. [PubMed ID: 34126091]. https://doi.org/10.1016/j.ejphar.2021.174245.
-
25.
Nezamoleslami S, Sheibani M, Mumtaz F, Esmaeili J, Shafaroodi H, Dehpour AR. Lithium reverses the effect of opioids on eNOS/nitric oxide pathway in human umbilical vein endothelial cells. Mol Biol Rep. 2020;47(9):6829-40. [PubMed ID: 32888132]. https://doi.org/10.1007/s11033-020-05740-9.
-
26.
Tewari D, Sah AN, Bawari S, Nabavi SF, Dehpour AR, Shirooie S, et al. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr Neuropharmacol. 2021;19(2):114-26. [PubMed ID: 32348225]. [PubMed Central ID: PMC8033982]. https://doi.org/10.2174/1570159X18666200429001549.
-
27.
Ahmed MA, Kamel EO. Involvement of H2 S, NO and BDNF-TrkB signalling pathway in the protective effects of simvastatin against pentylenetetrazole-induced kindling and cognitive impairments in mice. Basic Clin Pharmacol Toxicol. 2020;127(6):461-76. [PubMed ID: 32562563]. https://doi.org/10.1111/bcpt.13457.
-
28.
Lv Q, Wang Y, Li Y, Zhao L, Gong Y, Wang M, et al. Rosuvastatin Reverses Hypertension-Induced Changes in the Aorta Structure and Endothelium-Dependent Relaxation in Rats Through Suppression of Apoptosis and Inflammation. J Cardiovasc Pharmacol. 2020;75(6):584-95. [PubMed ID: 32205566]. [PubMed Central ID: PMC7266002]. https://doi.org/10.1097/FJC.0000000000000828.
-
29.
Gu Y, Zhu D. nNOS-mediated protein-protein interactions: promising targets for treating neurological and neuropsychiatric disorders. J Biomed Res. 2020;35(1):1-10. [PubMed ID: 33402546]. [PubMed Central ID: PMC7874267]. https://doi.org/10.7555/JBR.34.20200108.
-
30.
Shao S, Xu M, Zhou J, Ge X, Chen G, Guo L, et al. Atorvastatin Attenuates Ischemia/Reperfusion-Induced Hippocampal Neurons Injury Via Akt-nNOS-JNK Signaling Pathway. Cell Mol Neurobiol. 2017;37(4):753-62. [PubMed ID: 27488855]. https://doi.org/10.1007/s10571-016-0412-x.
-
31.
Chen T, Wang Y, Zhang T, Zhang B, Chen L, Zhao L, et al. Simvastatin Enhances Activity and Trafficking of alpha7 Nicotinic Acetylcholine Receptor in Hippocampal Neurons Through PKC and CaMKII Signaling Pathways. Front Pharmacol. 2018;9:362. [PubMed ID: 29706890]. [PubMed Central ID: PMC5906710]. https://doi.org/10.3389/fphar.2018.00362.