Abstract
Background:
Exogenous electrical stimulation of the skin may mimic its endogenous bioelectric currents. In this study, a combination of direct current (DC) and magnetic field (MF) was investigated in an excision wound model in rats.Methods:
A circular wound was created on the posterior of the neck, and an electrode was fixed in the wound center. Rats were divided into sham, DC (600 µA), MF (~0.8 T), and magnet-direct current (MDC) groups. The study was conducted in 14 days with 20-min treatment daily.Results:
The DC and MDC groups had higher healing percentages (P < 0.01) with mean differences of -13.42 and -15.63, respectively. Direct current on days 2, 5, and 6, and MDC on days 8, 9, 10, 11, 12, and 13 showed higher wound closing. In the DC-treated group, angiogenesis was improved on day 7. In MDC-treated rats, angiogenesis and fibroplasia were improved on day 13. The MF and MDC groups had lower granulation thicknesses on day 7. Granulation thickness increased on day 13 in the MF and MDC groups, while it decreased in the DC group. Direct current treatment improved healing in the first half of the study period, whereas MDC enhanced it in the second half, overtaking DC. From day 7, the magnet group's healing started to overtake the control group slightly in the last four days.Conclusions:
To accelerate wound healing, we suggest applying DC in the first days of wounding and MDC in the following days.Keywords
1. Background
The skin has an endogenous electrical potential regarding to its outer surface and it is always electronegative respecting the inner layers (1, 2). Several mechanisms intervene in producing the skin potential, eg, epidermal membranes, skin battery, sweat glands, and diffusion and dynamic pumping (2, 3). Nevertheless, following tissue damage, a current of injury is generated that is assumed to initiate biological repair, and consequently, the positive potential of the wound site diminishes with time as the wound surface shrinkages (2, 3). The current of injury is an electric field with less than 1 mA intensity, extending to a peri-wound radius of 2 - 3 mm, and the gradient gradually weakens from 140 to 0 mV/mm (4).
Of all physical modalities, ample evidence have stated using the electrical stimulation (ES) (5-8). Electrical stimulation has been widely used as a physical agent in medicine and rehabilitation, and this suggests that the wound endogenous bioelectric current exerts an adjusting role in the process of wound healing. Further, exogenous ES may mimic the endogenous current (8-11). A direct current (DC) of low intensity, which mimics the natural electrical field, probably stimulates wound healing by returning the current of injury in chronic circumstances (12). The current of injury appears to decrease intensity over time (13), leading to a plateau in tissue healing. Therefore, the external application of electrical current has been provided to promote the phases of repair and regeneration. Besides, ES increases the proliferation and migration of angiogenic elements (14) into the wound sites (15-17) and enhances skin perfusion (18, 19).
Anodal microamperage DC-ES may advance wound closing, speeding up the endogenous bioelectric happenings of the wound and attracting the epithelial cells. The endogenous electric conditions were strictly averaged by external ES once the wound surface was covered with the positive stimulation electrode, whereas the negative electrode was placed to the tissue surrounding the wound (20). Electrical stimulation using low-intensity currents is compatible with endogenous currents that act at the cellular level, accelerating wound healing and improving the quality of scar tissue (15). Using different amplitudes and frequencies, electrical stimulation promotes cellular modifications and tissue responses in experimentally induced wounds (21-23).
Another popular modality is magnet therapy, which promotes wound healing probably by reducing pain, speeding up healing time, and scar strength (24, 25). Stimulation with MF can trigger the majority of the processes participating in healing the bone and soft tissue. In several animal models, the stimulating impacts of MF on the healing of fractures, wounds, and pressure ulcers have been proven (5, 26). Static magnetic field (SMF) and pulsed electromagnetic field (PEMF), as non-invasive interventions, may exert substantial curative influences (27-29). Various animal experiments have established that SMF could promote the healing of several tissues, skin wounds, and nerve injuries (30-32). In numerous musculoskeletal injuries and post-surgical, post-traumatic, and long-lasting wounds, MFs are documented as a modality, lowering edema and a chief therapeutic element in speeding up pain relief, which sequentially contributes to healing. Animal investigations (in vivo) and cellular and membrane research (in vitro) propose that magnetic stimulation would enhance the healing processes (33-35). An externally applied, low-power SMF increased the rate of secondary wound healing. Wounds in the magnet group healed significantly faster (31). Studies have investigated the electromagnetic induction of controllable rotation in small-scale liquid flow and rotational behavior of an electrically conductive liquid under the application of external electric/magnetic fields. As experimental procedures and outcomes apply to somewhat varied situations, especially the bulk fluid, they can be employed to develop novel designs for manipulating the fluid flow in many essential areas, including biology (36).
2. Objectives
In the current experiment, we sought to investigate the effects of an externally employed, low-power, static magnetic field combined with direct electrical stimulation to possibly facilitate wound healing in an acute rat model.
3. Methods
3.1. Animals
Adult male Wistar rats (200 ± 20 g; 2 - 3 months old) were obtained from the animal house of the Pasture Institute of Iran. The animal room was maintained under controlled conditions (temperature 24 - 27°C and humidity 60 - 65% with a 12:12 h light: dark cycle). The Institutional Animal Ethics Committee of Tehran University of Medical Sciences approved all the experimental procedures per the National Institutes of Health for Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Thirty-two male rats were randomly divided into four groups of eight: Sham-operated group receiving no actual treatment, DC-treated group exposed to DC, MF-treated group exposed to a magnetic field, and the MDC group exposed to magnetic field and DC.
3.2. Excision of Wound Model
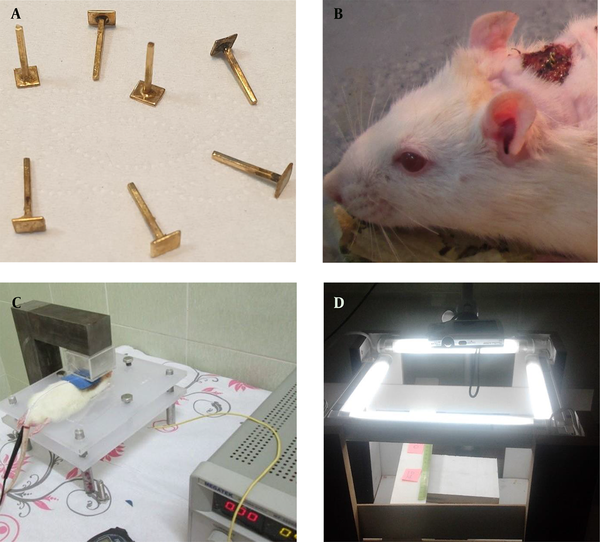
On day 0, rats were anesthetized with an intraperitoneal (i.p.) injection of ketamine 10% (50 mg/kg) and xylazine 2% (10 mg/kg). Their back hairs were removed by anti-allergic Veet hair removal cream (Reckitt Benckiser), and the skin was disinfected with 70% alcohol and rinsed with saline. A circular full-thickness excision wound (~300 mm2 area and 2 mm depth) was created on the posterior surface of the rat neck (37), and a gold electrode was fixed in the center of the wound (Figure 1A and B). The wounds were covered with sterile gauze for the first 24 hours to achieve hemostasis, while no topical administration was done throughout the study. Accordingly, the rats were anesthetized daily with minimal doses of ketamine and xylazine.
The setups and systems employed in the study. The gold bar electrodes (A) were implanted in the center of the wounds (B). The MDC setup consists of a neodymium-based permanent magnet and a manual electrical stimulator (C). The camera setup (D).
3.3. Treatment Procedures
Two electrodes were employed for the transmission of DC. The positive electrode (cathode) was externally applied around the wound, and the negative gold electrode (anode) was fixed permanently in the center of the wound with suture strings (Figure 1A and B). Nevertheless, this setting was reversed on days 4 - 13, and a radial current was expected inside the wound area. The implanted bar electrodes were made from gold with a tip surface of 0.25 cm2 contact area to prevent any tissue damage resulting from oxidation. Notably, the authors designed the electrodes exclusively for this study (Figure 1A).
This study was conducted in 14 days, and the animals received 20-min treatment for 12 consecutive days. In DC stimulation, a gold electrode was used as anode and a sticky circular pad electrode as the cathode in the first three days, and vice versa in the next 10 days. A medical lubricant gel facilitated the current transmission in the circular pad, which was on the contact area around the wound. For electrical stimulation, a manual stimulator (Physiotonus Microcurrent, Bioset, Rio Claro, Sao Paulo, Brazil) was employed. The system was fixed to microgalvanic-continuous mode with an intensity of 600 µA (2, 38, 39). To provide the magnetic field, we made a neodymium-base permanent magnet setup to provide a powerful virtually uniform magnetic field (~ 0.8 Tesla), and the animals were put between its two poles (Figure 1C). The sham-operated group received the same handling and electrode placement, but no current or magnetic field was applied.
3.4. Morphometric Study
Digital photographs were captured from the wound daily using a digital camera (Canon PowerShot A2500) fixed in a camera box setup (Figure 1D) to measure the wound closing rate. Magnification of the photos was calibrated by a standard ruler fixed close to the wound site. Each day, the wound area was measured by analyzing each photo with image processing software ImageJ (version 1.46 r).
3.5. Histopathological Study
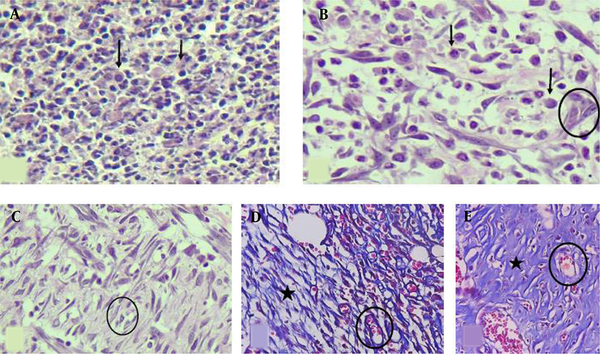
The histological analysis included the measurements of inflammation, angiogenesis, fibroplasia, and granulation tissue thickness in tissue sections taken on experiment days 7 and 13 (Figure 2). Animals were sacrificed by an overdose of ether, four rats per group on day 7 and four rats per group on day 13 post-wounding. Full-thickness skin samples and their surrounding areas, including the scab and approximately 5 mm margins of the surrounding tissue, were excised from the wound area and fixed in buffered formaldehyde (10%). Representative sections (5 μm) from the mid-site of the wound were stained by hematoxylin and eosin (H & E). The slides were examined blindly by a pathologist. Each slide was given a score ranging from 0 to 3 (absent: 0, mild: 1, moderate: 2, severe: 3) (Figure 2), according to the method described (40).
H & E-stained sections showing (A) severe inflammation (score 3), no angiogenesis or fibroplasia (score 0), (B) moderate inflammation (score 2), mild angiogenesis (score 1), and edema without obvious fibroplasia, (C) moderate inflammation, moderate angiogenesis, and mild fibroplasia, (D) Masson’s trichrome stain shows mild inflammation, moderate angiogenesis, and mild fibroplasia, (E) Masson’s trichrome stain shows minimal inflammation, mild angiogenesis, and marked fibroplasia (400X). Black arrows: inflammatory cells, Circles: new vessels (angiogenesis), Star area: collagen deposition (fibroplasia).
3.6. Statistical Analysis
We used SPSS version 18 software for data analysis. A two-way repeated-measures Analysis of Variance (ANOVA) for one factor (time) was used to test differences in wound diameters (41). One-way ANOVA was employed for between-group differences during the study. For qualitative pathological data, statistically significant changes in the mean values were analyzed by the chi-square and Pearson correlation qualitative test. The data were represented as mean ± SEM, and P < 0.05 indicated the significant differences.
4. Results
4.1. Healing Percentages
The total number of points inside the wound borders was counted, and the healing percentages are demonstrated in Figure 3. Two-way repeated-measures ANOVA on factor time showed that 13 days post-wounding, a decrease in wound area over time happened in various degrees. The within-subject effect was determined with Greenhouse-Geisser for time and time * group, showing the changes were significant (F = 68.33, P < 0.000 and F = 3.95, P < 0.001, respectively). The between-subject effect showed that the groups were statistically different (F = 6.59, P < 0.005). In a multivariate test, the Tukey post hoc test demonstrated that the DC group was significantly different from the control group and had a higher rate of wound closing (P < 0.01, mean difference= -13.42). Similarly, the MDC group showed a significant difference from the control group (P < 0.01, mean difference = -15.63).
Effects of DC, M, and MDC treatments on healing percentages of wounded rats showing the wound surface areas on a 14-day course of the study compared to the control group (sham-operated). *P
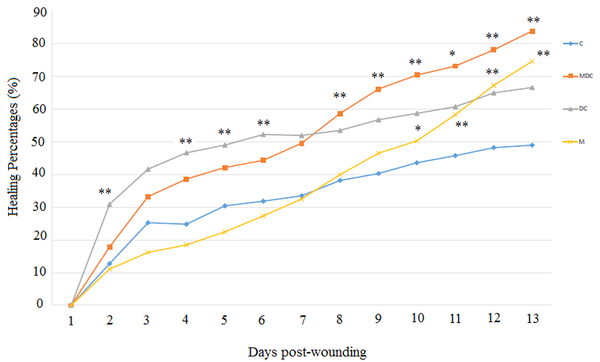
The DC exposure improved wound healing in the first half of the study (day 7) compared to the control group. One-way ANOVA revealed that significant changes occurred on days two [(F (3, 30) = 6.25, P < 0.004], four [(F (3, 31) = 7.744, P < 0.003], five [(F (3, 32) = 9.948, P < 0.006], and six [(F (3, 32) = 8.927, P < 0.003]. The combination of DC and M (MDC) improved wound healing percentages in the second half of the study (day 13). One-way ANOVA revealed that significant changes occurred on days eight [(F (3, 15) = 6.941, P < 0.007], nine [(F (3, 15) = 5.853, P < 0.009], 10 [(F (3, 15) = 5.665, P < 0.006], 11 [(F (3, 15) = 6.353, P < 0.012], 12 [(F (3, 15) = 8.391, P < 0.005], and 13 [ (F (3, 15) = 9.974, P < 0.003] (Figure 3). Moreover, the MF treatment significantly improved wound healing percentages in the last four days. One-way ANOVA revealed that significant changes occurred on days 10 [(F (3, 15) = 5.665, P < 0.03], 11 [(F (3, 15) = 6.353, P < 0.007], 12 [(F (3, 15) = 8.391, P < 0.002], and 13 [(F (3, 15) = 9.974, P < 0.001] (Figure 3).
4.2. Microscopic Outcomes
Microscopic images are shown for days 7 and 13 in Figure 4.
H & E-stained slides showing the skin microscopic changes. On day 7 of sampling: The skin tissue with (A) moderate inflammation, moderate angiogenesis, and mild fibroplasia in the control group, (B) moderate inflammation, marked angiogenesis, and mild fibroplasia in the DC-treated group, (C) severe inflammation, moderate angiogenesis, and minimal fibroplasia in the magnet group, (D) mild inflammation, moderate angiogenesis, and moderate fibroplasia in the MDC group. On day 13 of sampling: The skin tissue with (E) mild inflammation, moderate angiogenesis, and mild fibroplasia in the control group, (F) moderate inflammation, marked angiogenesis, and mild to moderate fibroplasia in the DC-treated group, (G) mild inflammation, moderate angiogenesis, and fibroplasia in the magnet group, H) mild inflammation, moderate angiogenesis, and marked fibroplasia in the MDC group (100×).
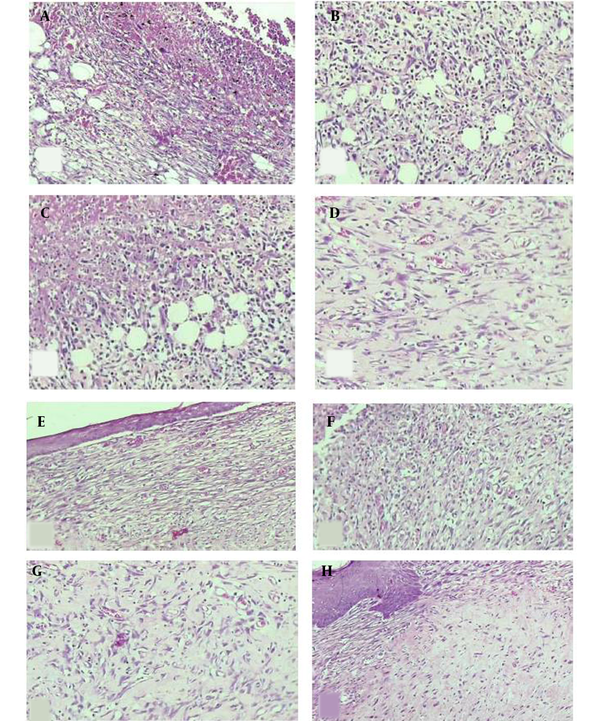
Day 7: Microscopic study of the skin tissue: (A) moderate inflammation, moderate angiogenesis, and mild fibroplasia in the control group, (B) moderate inflammation, marked angiogenesis, and mild fibroplasia in DC group, (C) severe inflammation, moderate angiogenesis, and minimal fibroplasia in MF group, (D) mild inflammation, moderate angiogenesis, and moderate fibroplasia in MDC group.
Day 13: Microscopic study of the skin tissue: (E) mild inflammation, moderate angiogenesis, mild fibroplasia in the control group, (F) moderate inflammation, marked angiogenesis, and mild to moderate fibroplasia in the DC group, (G) mild inflammation, moderate angiogenesis, and fibroplasia in the MF group, (H) mild inflammation, moderate angiogenesis, and marked fibroplasia in the MDC group (Figure 4).
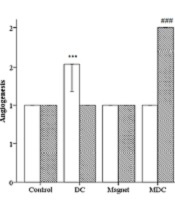
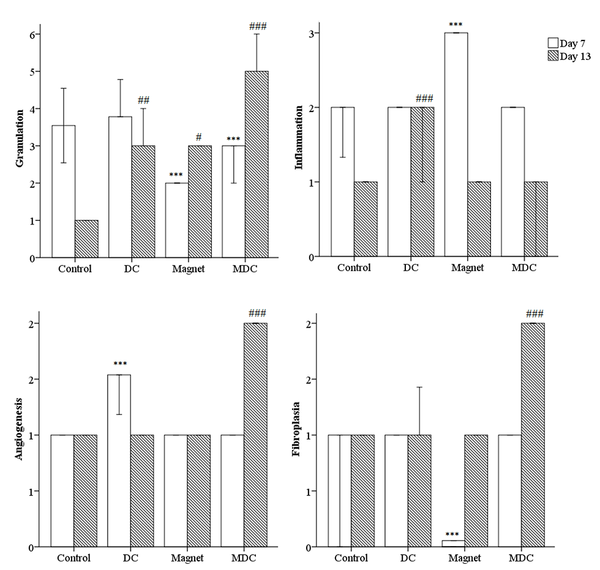
The descriptive analysis of histopathology outcomes demonstrated changes in the qualitative factors compared to the control group (Figure 5). On day 7, MF-exposed rats had higher inflammation [(Pearson chi-square = 8.000a, df: 9), P = 0.000]. On the other hand, the angiogenesis was improved in DC-exposed rats [(Pearson chi-square = 12.000a, df: 12), P = 0.000]. Fibroplasia was lower in MF-exposed rats [(Pearson chi-square = 16.000a, df: 18), P = 0.000]. On day 13, DC-exposed rats had higher inflammation [(Pearson chi-square = 15.000a, df: 15), P = 0.000]. On the other hand, angiogenesis and fibroplasia were improved in MDC-exposed rats [(Pearson chi-square = 15.000a, df: 15), P = 0.000 and (Pearson chi-square = 21.000a, df: 18), P = 0.000, respectively]. In addition, one-way ANOVA revealed significant changes in the granulation thickness on days 7 and 13 of the study. On day 7, both MF and MDC groups had lower granulation thicknesses than the control group [F (3, 16) = 2.000, P = 0.000]. On day 13, the granulation thickness was increased in both MF and MDC groups [F (3, 16) = 17.000, P = 0.047 and F (3, 16) = 17.000, P = 0.000, respectively]. Conversely, the granulation thickness was reduced in the DC group [F (3, 16) = 17.000, P = 0.007] (Figure 5).
Microscopic scoring to measure the qualitative factors including inflammation, angiogenesis, fibroplasia, and quantitative factor granulation. *** (P < 0.001) significantly different from day 7 of the control group. # (P < 0.05), ## (P < 0.01) and ### (P < 0.001) significantly different from day 13 of the control group.
5. Discussion
The current experiment investigated the effects of a permanent MF, DC, and their combination (MDC) on cutaneous wound healing in an acute rat model. Exposure to MDC increased the rate of healing. Notably, DC accelerated wound healing in the first week post-wounding; however, MDC and MF therapies were more effective in the second week. According to the pathological evaluations, the effect of MDC was significant in the second half of the study.
In line with our study, the effects of sensory (DC, 600 µA) and motor (pulse duration 300 µs., 100 Hz, 2.5 - 3.0 mA) intensities of cathodal ES current were investigated in the full-thickness wound in rats. An active electrode (1 × 3 cm) was located on the incision wound area and a passive electrode (2 × 4 cm) on the reverse side. The experimental groups received ES every other day for one hour (38). The microcurrent stimulation (10 μA) promoted tissue restoration and positively influenced the freshly shaped tissue area, numbers of fibroblasts and newly shaped vessels, and the epithelial thickness in rats (42).
Frequency-modulated ES enhanced the plasma levels of the vascular endothelial growth factor (VEGF) in both diabetic and non-diabetic subjects (43). Likewise, cathodal DC (CDC) raised the VEGF and nitric oxide (NO) plasma concentrations in diabetic foot ulcers (DFUs) (44). Recently, low-intensity CDC was employed three days per week in the wound liquid from ischemic DFUs in a randomized, placebo-controlled trial. The active electrode was the negative DC pole (cathode). Besides, ES with CDC was applied on the wound area using the active electrode placed adjacent to the proximal ulcer edge. The passive electrode was placed 20 cm proximal to the cathode electrode. It was recommended that ES in ischemic DFUs would be a promising approach in promoting angiogenesis (45).
Moreover, the effects of anodal and cathodal-pulsed ES, a unidirectional pulse current of 300 - 600 µA, 80 pps, and 0.3 ms pulse for one hour/day, on wound healing in guinea pigs demonstrated that cathodal and anodal stimulations enhanced wound closure rates. Irrespective of its polarity schedule, ES assisted in wound healing; nevertheless, anodal stimulation for the first three days and cathodal stimulation for the remaining days would enhance tissue repair (46). Anodal microamperage DC ES was suitable for improving acute skin wounds in guinea pigs. There was a positive correlation between wound closing in the skin and return of the injury potential to the normal level (2).
Like our investigation, previous studies varied the type of treatment polarity throughout the wound healing process (22, 47-49). The negative polarity had antibacterial effects (50, 51), and the positive polarity augmented the migration and proliferation of epithelial cells (5, 12, 52). Regarding the antibacterial property of negative polarity and the epithelialization impact of positive polarity, the negative polarity was employed for the first three days and the positive polarity for the remaining days (22, 47).
Regarding magnet therapy, an externally applied, low-power SMF increased the rate of secondary healing while a 23 gauss magnet (2 × 2 cm) was located over the wound on the posterior of the rat body (31). Their research is virtually consistent with our outcome. In another investigation, the influence of MF with a moderate-intensity gradient was investigated on diabetic wounds in rats. The findings showed that SMF exposure (180 mT) speeded wound closure (53). Likewise, the therapeutic effects of SMF (230 mT intensity) on cutaneous wound healing in diabetic rats were shown (54). In addition to accelerated wound healing, MF modalities raised local blood flow in the treated area, improving the ischemic tissue (55, 56). The effect of an MF (magnetohydrodynamic blood flow) with varying intensities in a cohort study of patients with coronary artery disease showed that externally applied MF may improve the hemodynamic perturbations in human coronary arteries (57).
In diabetic rats, a combination of EC and MF, tremendously low frequency pulsed electromagnetic fields (ELF PEMFs) 20 Hz, 4 ms, 8 mT for one hour daily, enhanced skin wound healing (58). This study was consistent with our experiment, except that they used a low-frequency alternating current (AC)-electromagnet with lower amplitudes in a chronic circumstance while we employed a permanent magnet with higher amplitudes.
Overall, the magnetic and electric stimulations have been connected to augmented collagen deposition, boosted ion transport, amino acid uptake, fibroblast migration, ATP, and protein synthesis, leading to a marked increase in protein and DNA synthesis rates after human fibroblast stimulation in tissue culture (59-61).
5.1. Limitations and Suggestions
We preferred to examine the physical modalities in chronic circumstances; however, we initiated a preliminary experiment to fulfill our systems and devices. Moreover, we were interested in employing an AC electromagnet to be able to tune the frequency and amplitude of the MF and also design a new geometry for the magnet. Further, it was favorable utilizing an AC to evaluate the effects of different frequencies. However, to have a more efficient magnetic hydrodynamic effect in the wound area, we needed to utilize a strong DC to provide a homogeneous circular rotation (while an AC generates a sequence of the clockwise and anti-clockwise rotations concerning the applied AC frequency). On the other hand, to perform a non-destructive healing procedure, we were restricted to the utilized current intensity. Furthermore, it was favorable to design a non-invasive electrode, from platinum or gold, with a thinner tip, without implanting it in the wound. Additionally, it would be ideal measuring the current of injury. Optimizing our treatment regimens, it would be possible to accelerate wound healing in cases where the body's natural healing mechanisms are not sufficient, for example, in older individuals and chronic wounds such as diabetic ulcers and pressure sores.
5.2. Conclusions
The DC treatment improved wound healing in the first half of the study and remained steady thereafter. In addition, MDC improved wound healing percentages in the second half. In the second week of the study, the effect of MDC overtook that of DC. Moreover, the magnet group started to overtake slightly the control group from day seven. However, the differences were statistically significant in the last four days. In this regard, DC may be employed to accelerate wound healing in the first days of wounding, while MDC may be started in the second week to acquire the optimum effect. Of note, the treatments probably assist in wound healing partly by inducing liquid flow in the wound area.
References
-
1.
Huttenlocher A, Horwitz AR. Wound healing with electric potential. N Engl J Med. 2007;356(3):303-4. [PubMed ID: 17229960]. https://doi.org/10.1056/NEJMcibr066496.
-
2.
Talebi G, Torkaman G, Firoozabadi M, Shariat S. Effect of anodal and cathodal microamperage direct current electrical stimulation on injury potential and wound size in guinea pigs. J Rehabil Res Dev. 2008;45(1):153-9. [PubMed ID: 18566934]. https://doi.org/10.1682/jrrd.2007.05.0068.
-
3.
Barker AT, Jaffe LF, Vanable JJ. The glabrous epidermis of cavies contains a powerful battery. Am J Physiol. 1982;242(3):R358-66. [PubMed ID: 7065232]. https://doi.org/10.1152/ajpregu.1982.242.3.R358.
-
4.
McGinnis ME, Vanable Jr JW. Voltage gradients in newt limb stumps. Prog Clin Biol Res. 1986;210:231-8.
-
5.
Alvarez OM, Mertz PM, Smerbeck RV, Eaglstein WH. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol. 1983;81(2):144-8. [PubMed ID: 6308102]. https://doi.org/10.1111/1523-1747.ep12543498.
-
6.
Brown M, Gogia PP. Effects of high voltage stimulation on cutaneous wound healing in rabbits. Phys Ther. 1987;67(5):662-7. [PubMed ID: 3495010]. https://doi.org/10.1093/ptj/67.5.662.
-
7.
Brown M, McDonnell MK, Menton DN. Electrical stimulation effects on cutaneous wound healing in rabbits. A follow-up study. Phys Ther. 1988;68(6):955-60. [PubMed ID: 3259701]. https://doi.org/10.1093/ptj/68.6.955.
-
8.
Vodovnik L, Karba R. Treatment of chronic wounds by means of electric and electromagnetic fields. Part 1. Literature review. Med Biol Eng Comput. 1992;30(3):257-66. [PubMed ID: 1453797]. https://doi.org/10.1007/BF02446963.
-
9.
Prentice WE. Therapeutic modalities in rehabilitation. McGraw Hill Professional; 2017.
-
10.
Hampton S, King L. Healing an intractable wound using bio-electrical stimulation therapy. Br J Nurs. 2005;14(Sup3):S30-2. https://doi.org/10.12968/bjon.2005.14.Sup3.18608.
-
11.
Nuccitelli R. A Role for Endogenous Electric Fields in Wound Healing. Sci Direct. 2003;58:1-26. https://doi.org/10.1016/s0070-2153(03)58001-2.
-
12.
Kloth LC, McCulloch JM. Promotion of wound healing with electrical stimulation. Adv Wound Caref. 1996;9(5):42-5.
-
13.
Jaffe LF, Vanable JW. Electric fields and wound healing. Clin Dermatol. 1984;2(3):34-44. https://doi.org/10.1016/0738-081x(84)90025-7.
-
14.
Bennett NT, Schultz GS. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am J Surg. 1993;165(6):728-37. https://doi.org/10.1016/s0002-9610(05)80797-4.
-
15.
Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds. 2005;4(1):23-44. [PubMed ID: 15860450]. https://doi.org/10.1177/1534734605275733.
-
16.
Park HJ, Rouabhia M, Lavertu D, Zhang Z. Electrical Stimulation Modulates the Expression of Multiple Wound Healing Genes in Primary Human Dermal Fibroblasts. Tissue Eng Part A. 2015;21(13-14):1982-90. [PubMed ID: 25873313]. https://doi.org/10.1089/ten.TEA.2014.0687.
-
17.
Rouabhia M, Park H, Meng S, Derbali H, Zhang Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS One. 2013;8(8). e71660. [PubMed ID: 23990967]. [PubMed Central ID: PMC3747189]. https://doi.org/10.1371/journal.pone.0071660.
-
18.
Gilcreast DM, Stotts NA, Froelicher ES, Baker LL, Moss KM. Effect of electrical stimulation on foot skin perfusion in persons with or at risk for diabetic foot ulcers. Wound Repair Regen. 1998;6(5):434-41. [PubMed ID: 9844163]. https://doi.org/10.1046/j.1524-475x.1998.60505.x.
-
19.
Peters EJ, Armstrong DG, Wunderlich RP, Bosma J, Stacpoole-Shea S, Lavery LA. The benefit of electrical stimulation to enhance perfusion in persons with diabetes mellitus. J Foot Ankle Surg. 1998;37(5):396-400. https://doi.org/10.1016/s1067-2516(98)80048-3.
-
20.
Karba R, Šemrov D, Vodovnik L, Benko H, Sˇavrin R. DC electrical stimulation for chronic wound healing enhancement Part 1. Clinical study and determination of electrical field distribution in the numerical wound model. Bioelectrochem Bioenerg. 1997;43(2):265-70. https://doi.org/10.1016/s0302-4598(96)05192-6.
-
21.
Bassett CA. Beneficial effects of electromagnetic fields. J Cell Biochem. 1993;51(4):387-93. [PubMed ID: 8496242]. https://doi.org/10.1002/jcb.2400510402.
-
22.
Bayat M, Asgari-Moghadam Z, Maroufi M, Rezaie FS, Bayat M, Rakhshan M. Experimental wound healing using microamperage electrical stimulation in rabbits. J Rehabil Res Dev. 2006;43(2):219-26. [PubMed ID: 16847788]. https://doi.org/10.1682/jrrd.2005.05.0089.
-
23.
Becker RO, Selden G, Bichell D. The body electric: Electromagnetism and the foundation of life. San Francisco Chronicle; 1985.
-
24.
Man D, Man B, Plosker H. The influence of permanent magnetic field therapy on wound healing in suction lipectomy patients: a double-blind study. Plast Reconstr Surg. 1999;104(7):2261-6. discussion 2267-8. [PubMed ID: 11149796]. https://doi.org/10.1097/00006534-199912000-00051.
-
25.
Szor JK, Topp R. Use of magnet therapy to heal an abdominal wound: a case study. Ostomy Wound Manag. 1998;44(5):24-9.
-
26.
Pilla AA. State of the art in electromagnetic therapeutics. Science; 1993. p. 17-22.
-
27.
Markov MS. Expanding use of pulsed electromagnetic field therapies. Electromagn Biol Med. 2007;26(3):257-74. [PubMed ID: 17886012]. https://doi.org/10.1080/15368370701580806.
-
28.
Ohkubo C, Okano H, Ushiyama A, Masuda H. EMF effects on microcirculatory system. Environmentalist. 2007;27(4):395-402. https://doi.org/10.1007/s10669-007-9082-z.
-
29.
Saunders R. Static magnetic fields: animal studies. Prog Biophys Mol Biol. 2005;87(2-3):225-39. [PubMed ID: 15556661]. https://doi.org/10.1016/j.pbiomolbio.2004.09.001.
-
30.
Bertolino G, de Freitas Braga A, de Oliveira Lima do Couto Rosa K, de Brito Junior LC, de Araujo JE. Macroscopic and histological effects of magnetic field exposition in the process of tissue reparation in Wistar rats. Arch Dermatol Res. 2006;298(3):121-6. [PubMed ID: 16773313]. https://doi.org/10.1007/s00403-006-0667-z.
-
31.
Henry SL, Concannon MJ, Yee GJ. The effect of magnetic fields on wound healing: experimental study and review of the literature. Eplasty. 2008;8.
-
32.
Kelleher MO, Al-Abri RK, Lenihan DV, Glasby MA. Use of a static magnetic field to promote recovery after peripheral nerve injury. J Neurosurg. 2006;105(4):610-5. [PubMed ID: 17044566]. https://doi.org/10.3171/jns.2006.105.4.610.
-
33.
Markov M. Thermal Vs. Nonthermal Mechanisms of Interactions between Electromagnetic Fields and Biological Systems. Bioelectromagnetics Current Concepts. Springer; 2006. p. 1-15. https://doi.org/10.1007/1-4020-4278-7_1.
-
34.
Morris C, Skalak T. Static magnetic fields alter arteriolar tone in vivo. Bioelectromagnetics. 2005;26(1):1-9. [PubMed ID: 15605401]. https://doi.org/10.1002/bem.20047.
-
35.
Okano H, Masuda H, Ohkubo C. Effects of 25 mT static magnetic field on blood pressure in reserpine-induced hypotensive Wistar-Kyoto rats. Bioelectromagnetics. 2005;26(1):36-48. [PubMed ID: 15605399]. https://doi.org/10.1002/bem.20052.
-
36.
Amjadi A, Nejati A, Sobhani SO, Shirsavar R. Electro/magnetically induced controllable rotation in small-scale liquid flow. arXiv. 2013.
-
37.
Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol. 2009;124(2):311-5. [PubMed ID: 19397984]. https://doi.org/10.1016/j.jep.2009.04.038.
-
38.
Asadi MR, Torkaman G, Hedayati M. Effect of sensory and motor electrical stimulation in vascular endothelial growth factor expression of muscle and skin in full-thickness wound. J Rehabil Res Dev. 2011;48(3):195-201. [PubMed ID: 21480094]. https://doi.org/10.1682/jrrd.2009.11.0182.
-
39.
Wood JM, Schallreuter KU, Jacobson WE, Sufit R, Newman J; Evans 3rd, et al. A multicenter study on the use of pulsed low-intensity direct current for healing chronic stage II and stage III decubitus ulcers. Arch Dermatol. 1993;129(8):999-1009. [PubMed ID: 8352625].
-
40.
Greenhalgh DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol. 1998;30(9):1019-30. https://doi.org/10.1016/s1357-2725(98)00058-2.
-
41.
Leffmann DJ, Arnall DA, Holmgren PR, Cornwall MW. Effect of microamperage stimulation on the rate of wound healing in rats: a histological study. Phys Ther. 1994;74(3):195-200. discussion 213-8. [PubMed ID: 8115453]. https://doi.org/10.1093/ptj/74.3.195.
-
42.
Mendonca FA, Passarini Junior JR, Esquisatto MA, Mendonca JS, Franchini CC, Santos GM. Effects of the application of Aloe vera (L.) and microcurrent on the healing of wounds surgically induced in Wistar rats. Acta Cir Bras. 2009;24(2):150-5. [PubMed ID: 19377785]. https://doi.org/10.1590/s0102-86502009000200013.
-
43.
Bevilacqua M, Dominguez LJ, Barrella M, Barbagallo M. Induction of vascular endothelial growth factor release by transcutaneous frequency modulated neural stimulation in diabetic polyneuropathy. J Endocrinol Invest. 2007;30(11):944-7. [PubMed ID: 18250616]. https://doi.org/10.1007/BF03349242.
-
44.
Mohajeri-Tehrani MR, Nasiripoor F, Torkaman G, Hedayati M, Annabestani Z, Asadi MR. Effect of low-intensity direct current on expression of vascular endothelial growth factor and nitric oxide in diabetic foot ulcers. J Rehabil Res Dev. 2014;51(5):815-25. [PubMed ID: 25509057]. https://doi.org/10.1682/jrrd.2013.08.0174.
-
45.
Asadi MR, Torkaman G, Hedayati M, Mohajeri-Tehrani MR, Ahmadi M, Gohardani RF. Angiogenic effects of low-intensity cathodal direct current on ischemic diabetic foot ulcers: A randomized controlled trial. Diabetes Res Clin Pract. 2017;127:147-55. [PubMed ID: 28371685]. https://doi.org/10.1016/j.diabres.2017.03.012.
-
46.
Mehmandoust FG, Torkaman G, Firoozabadi M, Talebi G. Anodal and cathodal pulsed electrical stimulation on skin wound healing in guinea pigs. J Rehabil Res Dev. 2007;44(4):611-8. [PubMed ID: 18247258]. https://doi.org/10.1682/jrrd.2007.01.0007.
-
47.
Brown M, Gogia PP, Sinacore DR, Menton DN. High-voltage galvanic stimulation on wound healing in guinea pigs: Longer-term effects. Arch Phys Med Rehabil. 1995;76(12):1134-7. https://doi.org/10.1016/s0003-9993(95)80122-7.
-
48.
Feedar JA, Kloth LC, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther. 1991;71(9):639-49. [PubMed ID: 1881954]. https://doi.org/10.1093/ptj/71.9.639.
-
49.
Taskan I, Ozyazgan I, Tercan M, Kardas HY, Balkanli S, Saraymen R, et al. A comparative study of the effect of ultrasound and electrostimulation on wound healing in rats. Plast Reconstr Surg. 1997;100(4):966-72. [PubMed ID: 9290665]. https://doi.org/10.1097/00006534-199709001-00020.
-
50.
Rowley BA, McKenna JM, Chase GR, Wolcott LE. The influence of electrical current on an infecting microorganism in wounds. Ann N Y Acad Sci. 1974;238:543-51. [PubMed ID: 4216282]. https://doi.org/10.1111/j.1749-6632.1974.tb26820.x.
-
51.
Barranco SD, Spadaro JA, Berger TJ, Becker RO. In Vitro Effect of Weak Direct Current on Staphylococcus Aureus. Clin Orthop Relat Res. 1974;100(5). https://doi.org/10.1097/00003086-197405000-00037.
-
52.
Castillo E, Sumano H, Fortoul TI, Zepeda A. The influence of pulsed electrical stimulation on the wound healing of burned rat skin. Arch Med Res. 1995;26(2):185-9.
-
53.
Jing D, Shen G, Cai J, Li F, Huang J, Wang Y, et al. Effects of 180 mT static magnetic fields on diabetic wound healing in rats. Bioelectromagnetics. 2010;31(8):640-8. [PubMed ID: 20607739]. https://doi.org/10.1002/bem.20592.
-
54.
Zhao J, Li YG, Deng KQ, Yun P, Gong T. Therapeutic Effects of Static Magnetic Field on Wound Healing in Diabetic Rats. J Diabetes Res. 2017;2017:6305370. [PubMed ID: 28459073]. [PubMed Central ID: PMC5385228]. https://doi.org/10.1155/2017/6305370.
-
55.
Bassett CA. Fundamental and practical aspects of therapeutic uses of pulsed electromagnetic fields (PEMFs). Crit Rev Biomed Eng. 1989;17(5):451-529.
-
56.
Markov MS. Biological Effects of Extremely Low Frequency Magnetic Fields. Biomagnetic Stimulation. Springer; 1994. p. 91-103. https://doi.org/10.1007/978-1-4757-9507-3_7.
-
57.
Javadzadegan A, Moshfegh A, Afrouzi HH, Omidi M. Magnetohydrodynamic blood flow in patients with coronary artery disease. Comput Methods Programs Biomed. 2018;163:111-22. [PubMed ID: 30119846]. https://doi.org/10.1016/j.cmpb.2018.06.007.
-
58.
Goudarzi I, Hajizadeh S, Salmani ME, Abrari K. Pulsed electromagnetic fields accelerate wound healing in the skin of diabetic rats. Bioelectromagnetics. 2010;31(4):318-23. [PubMed ID: 20082338]. https://doi.org/10.1002/bem.20567.
-
59.
Dini L, Abbro L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron. 2005;36(3):195-217. [PubMed ID: 15725590]. https://doi.org/10.1016/j.micron.2004.12.009.
-
60.
Okano H, Masuda H, Ohkubo C. Decreased plasma levels of nitric oxide metabolites, angiotensin II, and aldosterone in spontaneously hypertensive rats exposed to 5 mT static magnetic field. Bioelectromagnetics. 2005;26(3):161-72. [PubMed ID: 15768432]. https://doi.org/10.1002/bem.20055.
-
61.
Rosch PJ, Markov MS. Bioelectromagnetic Medicine. Informa Healthcare; 2004. https://doi.org/10.3109/9780203021651.