Abstract
Keywords
Lie Deliberate Deception Detection Brain Imaging Functional Magnetic Resonance Imaging (fMRI) Neural Activity
1. Background
Lying may refer to a word or a sentence replaced with the truth to seduce others (1). Individuals sometimes lie about their actions in everyday life. Telling a lie requires knowing the truth, suppressing the potentially accurate response, and then producing a novel response (2-4). In other words, lying is a complicated behavior encompassing multiple cognitive processes, including memory (5), inhibition (6, 7), attention (8), the theory of mind (9), and task switching (10).
Due to the rising demands for evident security, criminal concerns in societies, and emerging requests to discover accurate approaches in many industrial, legal, clinical, and justice settings, introducing meticulous and noninvasive lie detection methods would be of great importance. Some methods, such as thermal imaging techniques, have been proposed to predict and detect deception using the candidate clues (11), facial expressions (12), electrodermal response (13), and polygraph (14). They use peripheral measures to detect anxiety rather than lie (15); however, such methods suffer from the lack of specificity and sensibility. Accordingly, their collected data are unreliable and receive no proper scientific support (16). These lie detection techniques were developed with regard to the wrongful fact indicating that liars are stressed and aroused because of their being afraid of lie detection. Investigators can catch liars, while careful liars have been successful in faking the test (17). In contrast, truth-tellers might be regarded as liars since they show arousal signs and are anxious (18).
Early studies on the brain basis of deception aimed to detect a deceptive response accurately, according to which some brain regions' involvement was proposed (1, 19-22). This so-called "the neural approach" could offer an unrivaled way to observe the brain itself during deceptive behavior. A better understanding of neural circuitry would improve detection and diagnosis/treatment methods and is beneficial in courts and industrial or security settings, and under medical conditions.
Over the past twenty years, studies using multiple neuroimaging methods to detect deception have examined the behavioral and functional anatomical correlates of deception and deceptive processes in the brain (1, 23). These studies spotted increased activation in several brain regions when individuals lie and do not tell the truth. Deception-related brain changes were mainly associated with increased frontal, temporal, and parietal activation. However, the activated regions differ widely in different studies, which might be caused by diverse tasks. Moreover, despite the existing knowledge about the deception's neural essence in the past two decades, new paradigms are recommended to cover some shortages. Most of the previous paradigms have been developed to instruct the subjects when and how they must lie, and they do not resemble the cost/benefit value of the real-world circumstances.
In the present study, we used blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) to investigate brain activation in a deliberate deception task. To this end, we designed it as a monetary game. BOLD fMRI is a technique frequently employed to study the function of the brain noninvasively (24). The advent of functional brain imaging has provided a unique ground to monitor the various brain regions during cognitive processes directly and identify brain regions specific to deception-related activations in addition to measuring arousal. Along with the low signal-to-noise ratio, fMRI studies, including the present study, only offer neural correlate results at a group, not at the individual level.
2. Methods
2.1. Participants
Thirty-three healthy right-handed unmedicated volunteers (10 male and 23 female), aged 20 - 55 years, participated in the fMRI experiments, of whom two female participants were later excluded. The participants were screened using a structured clinical interview for DSM-V axis i disorders (25). Informed consent was obtained from all participants under the guidelines developed by the local Ethics Committee (Code: IR.TUMS.VCR.REC.1396.2312). We acquired a medical history, a pre-MRI screening form, and a handedness test (26). All participants were native Iranian Farsi speakers. They were screened and excluded for a medical or psychiatric illness, pregnancy, or the consumption of medications or drugs. Furthermore, the participants were allowed to study the questions to be mentioned during the imaging phase carefully.
During informal interviews conducted after the test period, most participants self-reported that they spared efforts to deceive the investigators under lie conditions. We welcomed the participants in our neuroimaging laboratory on the test day, as investigators apprised them about a previously prepared deception detection informative presentation. The participants were recruited from the university community and had a Bachelor's degree or higher levels of education.
2.2. Deception Detection Paradigm
We used a modified guilty knowledge task (GKT) (27) to set up our deception detection paradigm based on a real-world events model and designed a win-lose game. A monetary reward was offered to the participants to deceive investigators when they were asked about their choice in the game during the neuroimaging session. Furthermore, we also spared efforts to make the paradigm closer to situations observed in judicial settings. Most judicial cases encompass three types of information: (1) Information about which both investigator and suspect know the truth (e.g., the suspect's gender. name, age, and others); (2) The investigator knows the truth; however, the suspect tries to hide and lie about it (e.g., were they in a particular place? They deny their presence at the place even though there is some evidence about their presence in that specific place); and (3) The investigator does not know the truth about specific information and tries to find whether the suspect lies or tells the truth. This type of information is the main goal of the investigation.
2.3. Imaging
A Siemens 3 Tesla MRI scanner with a 64-channel head coil was used for brain mapping (PRISMA; 2016). The T2*-weighted images were collected for functional imaging based on the BOLD contrast by whole-head coverage. The sequence was a spin-echo, single-shot Echo-planar Imaging (EPI) sequence (TR = 3000 ms, TE = 30 ms, FOV = 192 mm2, flip angle = 90º, matrix size = 64 × 64, & slice gap = 0 mm) and acquired 40 slices with isotropic resolution (3 × 3 × 3 mm3). Before performing the EPI scan, a structural T1-weighted three-dimensional scan was obtained using a gradient echo sequence (TR = 1800 ms, TE = 3.44 ms, TI = 1100 ms, voxel size = 1 × 1 × 1 mm3, matrix size = 256 × 256, flip angle = 7°, FOV = 256 × 256 mm2, slice thickness = 1 mm, & slice gap = 0 mm). All data were anonymized before processing.
2.4. fMRI Paradigm
2.4.1. Phase 1: Informational Scenario
We prepared an informational written scenario about the instruction of the game-format task and submitted it to the volunteers. This written text of the proposed scenario is as follows: We ask you to play an insincere game. There are 10 cups in the side room, and each cup contains a piece of paper with a specific digit on it. Numbers are 10000, 20000, 30000, 40000, and 50000. The numbers are repeated twice in the cups, implying that there are two cups containing 10000, two cups with 20000, two cups with 30000, two cups with 40000, and two cups with 50000 (Figure 1). Each number represents its monetary value in Tomans (the official currency of Islamic Republic of Iran), and all cups are placed randomly on a table. We do not know which cup contains what number. You should choose just one cup, take the paper, and keep it until the task ends. In Phase 2, we will ask questions about the number you took before scanning your brain activity alterations. If we find the number, you will not receive any reward. Still, if you succeed in deceiving us about your number we fail to recognize the selected number accurately. In that case, you will receive a monetary cash reward ten times as much as your selected number (at this time, you can show your selected paper).
Each cup contains a piece of paper on which we wrote a specific number, including 10000, 20000, 30000, 40000, and 50000. The numbers are repeated twice among cups. Each number represents its monetary value in Tomans. We do not know which cup contains what number. The participants should choose just one cup, take the paper, and keep it until the task ends.
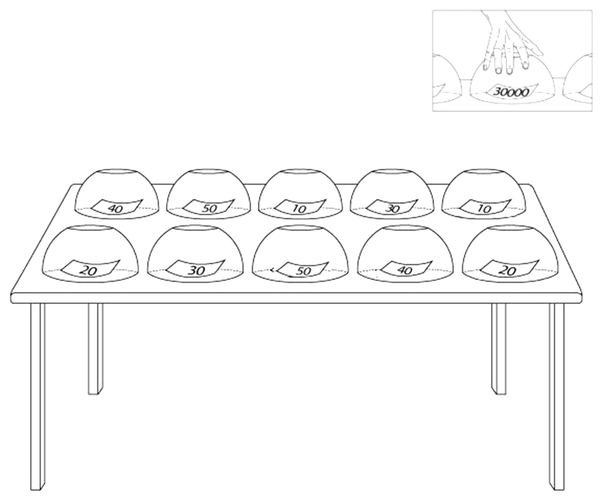
The participants were requested to spare their efforts to lie so that the researchers could not detect whether they were lying or telling the truth. Then, we guided the participants to the side room, where they could select a cup from ten cups and take the paper hidden under the cup. They were not allowed to look at what was hidden under the other cups.
2.4.2. Phase 2: Introduction to the Imaging Procedure
After choosing their piece of paper and before initiating the imaging process, we show them a computer-based schematic reconstruction of what they are expected to face in the MRI machine and how they can communicate with us and respond to the visual questions via the response box.
2.4.3. Phase 3: Neuroimaging
Following the introduction phase, we guided the participants to the imaging room. They were wearing MR-compatible glasses to watch continuous slides showing them a certain number among 10000, 20000, 30000, 40000, and 50000. They could respond to the asked questions via the response box, whether they had or did not have the presented number (Figure 2). The slides were presented to the participants during the MRI using an optic MR-compatible goggle.
If we find out which number the participant has, he/she will not receive any prize. However, if they succeed in deceiving us regarding their number, they will receive a tenfold cash reward. The design started with a cross-slide. Afterward, a number-slide representing one of the previously mentioned numbers is shown randomly. After that, there is another cross-slide and then another number. During this procedure, the participant uses the response box and answers either Yes (i.e., I have the presented number) or No (i.e., I do not have the presented number)
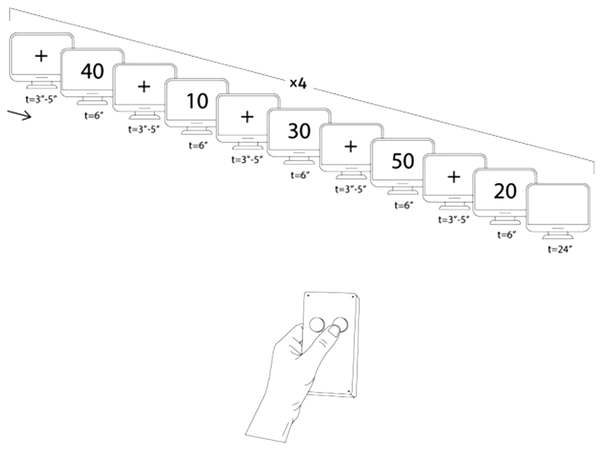
The block-design fMRI task: Figure 2 presents a schematic illustration of the block design. The design started with a cross-slide (t = 3, 4, 5 s randomly). Following the presentation of the cross-slide, a number-slide showing one of the previously mentioned digits was also presented randomly (t = 6 s). The cycle was repeated with another cross-slide and then another number. During this procedure, the participants used the response box and answered either Yes (i.e., I have the presented number) or No (i.e., I do not have the presented number). When all numbers (namely 10000, 20000, 30000, 40000, and 50000) were shown once, a 24-second rest (a blanket page slide) was run. Investigators repeated the procedure four times, indicating 20 conditions of lying or truth-telling in general.
2.4.4. Lie and Truth Conditions
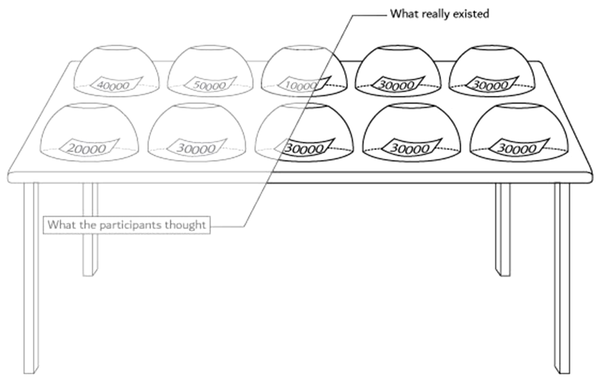
We hid one piece of information about the task and did not tell the truth about it to the participants. All cups contained 30000-Toman paper; however, the participants thought the numbers were from 10.000 to 50.000 as they had been informed (Figure 3). When the participants were asked about the number they had received in the room, the investigators knew that they had 30000-Toman papers, and that they would deny the number (Information Type II). Moreover, each subject tried to convince us that they took another number (e.g., 10.000 Tomans) and told the second lie when they saw the number slides. The third scenario included the truth condition because the subject did not have the number, and it was a truthful claim (Information Type I). In the beginning, we spared efforts to predict the second lie (Information Type III) based on a pattern of the same lie and the exact truth conditions. However, due to the low signal-to-noise ratio, we were limited to analyze the truth and lie scenarios separately.
All cups contained 30.000-Toman papers; however, the participants thought the numbers were from 10.000 to 50.000 as they had been informed.
2.5. Data Analysis
Preprocessing: Data preprocessing was performed in FSL (the FMRIB Software Library) v5.0.81 (28). First, motion correction was performed using MCFLIRT (29). Then the images were spatially normalized to the T1-weighted MNI (Montreal Neurological Institute) template using the linear FLIRT (29, 30). This procedure was performed in two steps: (1) the fMRI images were registered to the individual’s T1-weighted structural image using the BBR algorithm; and (2) the high-resolution T1-weighted image was registered to the standard MNI template using a 12 DOF linear transformation. These two steps were mixed into a registration step in which the EPI images were spatially registered to the MNI space. We manually inspected the performance of this step to guarantee a valid registration. Finally, spatial smoothing was performed using a Gaussian kernel with a 5-mm full-width at half maximum. Structural images were skull-stripped using BET (31) and segmented into white matter, gray matter, and cerebrospinal fluid using FAST (Jenkinson, Pechaud et al. 2005). The individuals' binarized tissue masks were then projected to the MNI space created earlier, and then they were averaged to generate the study-specific templates of different tissue types.
General linear model: The statistical analysis of the fMRI time series was performed using FILM (FMRIB Improved Linear Model) to validate the statistical approaches and make them maximally efficient, and a z-score was estimated for each corresponding BOLD signal. Next, we performed cluster thresholding to reveal the significantly activated clusters; the clusters with a z-value > 2.6 (= P < 0.001) were considered significantly activated. The following three contrasts were tested in this phase: (1) lie, (2) truth, and (3) lie minus truth. Higher-level analyses were later performed using FMRIB's local analysis of mixed effects for all the three contrasts (P < 0.001).
3. Results
The study's significant findings in the two contrasts of interests (namely lie and lie minus truth) are demonstrated in Table 1 and described below.
Increased Activation Areas
| Variables | Regions | BA | NI Coordinates | Cluster Size (Voxels) | Z-Value | ||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
| Right dorsolateral prefrontal cortex | 9 | 45 | 26 | 32 | 24 | 3.68 | |
| Right dorsomedial prefrontal cortex | 10 | 4 | 50 | 31 | 38 | 3.91 | |
| Lie | Right inferior parietal lobule | 40 | 36 | -48 | 45 | 34 | 4.16 |
| Minus | Right inferior frontal gyrus | 45/47 | 52 | 20 | 0 | 16 | 4.07 |
| Truth | Left inferior frontal gyrus | 45/47 | -47 | 20 | 0 | 44 | 3.86 |
| Right anterior cingulate cortex | 12 | 26 | 27 | 14 | 3.96 | ||
| Right dorsolateral prefrontal cortex | 10 | 45 | 26 | 32 | 49 | 4.00 | |
| Lie | Left dorsolateral prefrontal cortex | 10 | 35 | 3.69 | |||
| Right inferior frontal gyrus | 45/47 | 54 | 18 | 0 | 38 | 3.86 | |
| Right anterior cingulate cortex | 24/32 | 8 | 16 | 48 | 55 | 3.99 | |
3.1. Lie Minus Truth (Increased Activation)
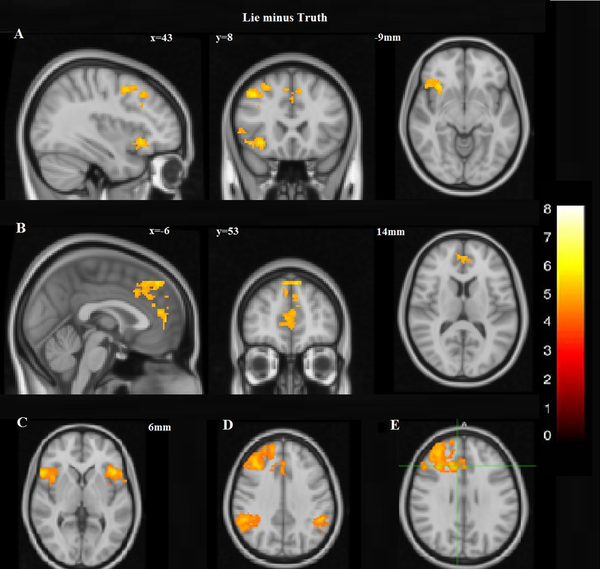
A dissociated pattern of activity was observed during deception within the prefrontal cortex. The pattern was distinguished by augmented activation having some parts of the dorsal regions of the medial prefrontal cortex (DMPFC) and the right lateral prefrontal cortex initiated from the lateral inferior frontal cortex (IFC) to the cranial parts of the dorsolateral prefrontal cortex (DLPFC). However, this expansive activation pattern was amalgamated by the focal activity of the frontal regions of MPFC (Figure 4A and B; Table 1). The overall excess in cognitive activity was associated with lying. Increased activations were also noticed in the inferior frontal gyrus (IFG) bilaterally (Figure 4C) and the posterior portions of the right middle temporal sulcus, lateral occipital gyri, and occipital fusiform, also the intervening intraparietal sulci and the inferior and right inferior parietal lobules. Increased activation was also presented in the inferior parietal cortex bilaterally. However, more significant activations were in the right portions of IPC (Figure 4D). Increased activity in the limbic system was also noticed during deception. Selective activations during deception were in the right anterior cingulate cortex (ACC) (Figure 4E).
Lie minus Truth: A, Increased activation, including some parts of the right lateral prefrontal cortex, extending from the lateral inferior frontal cortex (IFC) to the superior portions of DLPFC as well as the dorsal portions of DMPFC; B, However, this broad activation pattern was also accompanied with the focal activation of the frontal polar portion of MPFC; C, In the neocortical areas, there was increased activation in the inferior frontal gyrus (IFG) bilaterally; D, More significant activation was observed in the right portions of IPC; E Selective activations during deception were in the right anterior cingulate cortex (ACC).
3.2. Lie (Increased Activation)
Increased activation encompassing some parts of the right lateral prefrontal cortex, initiating from the inferior frontal cortex (IFC) to the cranial parts of DLPFC, was presented bilaterally. There was also increased activation in the right IFG and right ACC.
4. Discussion
Deception is considered a complicated process requiring special cognitive efforts (9). A successful liar foremost specifies the truth and then recruits cognitive orders in subduing the ongoing demand to make an honest reaction (32). This process is commonly referred to as cognitive control, one of whose essential elements is response inhibition behavior. Several studies on lying have reported the involvement of brain portions as a factor in charge of cognitive control (8, 33, 34). This study used fMRI to dissociate neural activity associated with deliberate deception during a monetary-rewarding task. We found new evidence showing the significant activations in different brain regions. Our findings imply a specific pattern of neural alterations in the prefrontal cortical activity underlying the deliberate deception process (lie-minus-truth). These findings are consistent with prior studies. Different regions in the frontal cortex are activated during cognitive control tasks, including deception. The collected data indicate that deliberate deception is augmented by the increased activation of the prefrontal cortex's dorsomedial and dorsolateral parts, right ACC, and bilateral IFG. This distinctive pattern may offer insights into the neural differentiation that may be essential to the deception process, indicating particular cognitive order encompassing inhibitory responses, decision-making in beneficial situations, conflict monitoring, increased cognitive load, shift and task planning, the spontaneous expression of a false statement as a lie, and intentional suppression of truth to mislead others with pseudo-fact. We distinguished regions probably engaged in deceptive responses (19, 32, 35). Some studies have indicated that deception activates neural systems underlying executive functions and is associated with neural activity in IFG), ACC, the dorsomedial prefrontal cortex, and DLPFC (15, 36-38).
ACC is significantly activated by spontaneous lying and is correlated with pretending of not- knowing the truth (32, 39, 40). Furthermore, ACC acts as a cognitive modulator in emotionally conflicting situations (41) and as a region belonging to the brain's error detecting system (42, 43). Dorsal ACC (BA 24, 32) also might be associated with conflict monitoring (44) and response inhibition. In this regard, ACC activation may be associated with dishonest responses, triggering emotional conflict when making dishonest decisions, and the truth blockade. We found similar activation in ACC under deliberate lying conditions that the participants’ dishonest decision-making (lying) seems to trigger emotional conflict.
The anterior and the dorsomedial prefrontal cortices exhibited a more robust response in value-based decision-making and profitable actions (45-48). Moreover, the DLPFC activation was also observed during demanding problem-solving tasks, conscious self-monitoring, and focused attention (49-51). Given the association between DLPFC activity with the inhibition of pre-potent impulses (52) and the discouragement of declaration of individual thoughts (53), these two facts conform to the truth-suppression context. The increased activity of DLPFC during lying is also in agreement with this developing prospect suggesting that DLPFC plays a role as an underpinning neural supporting decision-making, organizing internally-motivated behavior, and cognitive control. The impairment of right DLPFC augmented unwanted behavior by lowering self-control (54, 55). Its dysfunction caused by an external intervention slowed up the speed of lie production. As a result, one could argue that lying is a manner of restricting intentional truthful responses under advantageous conditions.
IFG is a part of the brain, which regulates inhibitory responses (56, 57) and is linked to risk-taking behavior (58). Previous studies also have documented that unlike the liars, the truth-tellers represent no increased activation in IFG, which might reflect their non-risky choices. IFG is one of those impaired brain regions in the PD patients (59). Given that the PD patients lack deceptive responses and cannot lie or deceive others, behavioral deficit is assumed to be caused by the damaged IFG and the non-evaluation of the risky decision. These findings implicate that the IFG may provide a neural marker for risk-taking and successful lying (60). Moreover, it was activated more in successful liars than in less-skilled ones (60).
We believe that the formidable dissociation of the increased activation of DLPFC, DMPFC, ACC, and IFG observed during the deliberate deception task is highly meaningful. Suppose that increased activity in DLPFC, DMPFC, ACC, and IFG serves as an index of conflict monitoring, inhibitory response, and value-based decision-making. In that case, deliberate deception seems to rely on the activation of the error detecting and truth-suppressing systems, which indicate determining the truth and then effortful insistence on suppressing the ongoing unveiling of deception-based emotional demand to make a truthful response. This finding is likely compatible with the dissociated model of prefrontal activity we scrutinized. Hence, deception may be associated with demeanors adapting to rules executed by DLPFC, DMPFC, ACC, and IFG without emotional conflict. To be more concise, deceptive behavior may result from the incorporation of conflicting-emotion regulation (ACC-mediated), risk-taking behavior (IFG-mediated), value-based decision making (DMPFC-mediated) with the inhibitory-response (DLPFC-associated) regulating deliberate deception. It is reasonable to investigate if activity modifications during deception, which are contracted by motor and limbic system changes, are initiated top-down and extend from PFC. Basically, these alterations might be consistent with implementing and encoding emotional or motor programs representing deliberate deception rather than demonstrating increased activity in the limbic and motor activity. Further, the engagement of the limbic system was envisioned in this study because of the obvious relationship between deliberate deception and emotion. The activation of the right Amygdala can be attributed to the emotional self-monitoring response related to deception, confirming the finding indicating these limbic activities during deception or deduced intense emotions.
4.1. Conclusions
The present study mainly aimed to detect the alterations of activity during deceptive responses. The variation of the brain regions involved in dishonest behavior was significant. Activation pattern, mainly occurring in the frontal and the limbic brain regions during deception, was compared to the pattern observed in the honest participants. This finding suggests that activity alterations are meaningful since the subject inhibits or recruits different brain parts to behave deceitfully. Increased activity in lying scenarios indicates that the relevant brain areas are activated simultaneously. The above-described activation brain regions eventually become dominant activity parts.
4.2. Limitations
In addition to the low signal-to-noise ratio in the fMRI studies, including the present study, such studies only offer neural correlate results at a group, not individual level. Moreover, we had to consider the time limitation to avoid cognitive loads. Furthermore, the collected data in this study was limited to the right-handed participants, and we did not know whether laterality in the brain regions was affiliated to dominant or non-dominant brain regions.
References
-
1.
Spence SA, Farrow TF, Herford AE, Wilkinson ID, Zheng Y, Woodruff PW. Behavioural and functional anatomical correlates of deception in humans. Neuroreport. 2001;12(13):2849-53. [PubMed ID: 11588589]. https://doi.org/10.1097/00001756-200109170-00019.
-
2.
Debey E, Ridderinkhof RK, De Houwer J, De Schryver M, Verschuere B. Suppressing the truth as a mechanism of deception: Delta plots reveal the role of response inhibition in lying. Conscious Cogn. 2015;37:148-59. [PubMed ID: 26397036]. https://doi.org/10.1016/j.concog.2015.09.005.
-
3.
Kireev M, Korotkov A, Medvedeva N, Masharipov R, Medvedev S. Deceptive but Not Honest Manipulative Actions Are Associated with Increased Interaction between Middle and Inferior Frontal gyri. Front Neurosci. 2017;11:482. [PubMed ID: 28912675]. [PubMed Central ID: PMC5583606]. https://doi.org/10.3389/fnins.2017.00482.
-
4.
Vrij A. Interviewing to Detect Deception. Eur Psychol. 2014;19(3):184-94. https://doi.org/10.1027/1016-9040/a000201.
-
5.
Abe N, Okuda J, Suzuki M, Sasaki H, Matsuda T, Mori E, et al. Neural correlates of true memory, false memory, and deception. Cereb Cortex. 2008;18(12):2811-9. [PubMed ID: 18372290]. [PubMed Central ID: PMC2583150]. https://doi.org/10.1093/cercor/bhn037.
-
6.
Walczyk JJ, Cockrell NF. To err is human but not deceptive. Mem Cognit. 2022;50(1):232-44. [PubMed ID: 34136972]. https://doi.org/10.3758/s13421-021-01197-8.
-
7.
Ekman P. Lie Catching and Microexpressions. In: Martin C, editor. The Philosophy of Deception. Oxford, UK: Oxford University Press; 2009. p. 118-36. https://doi.org/10.1093/acprof:oso/9780195327939.003.0008.
-
8.
Gibbons H, Schnuerch R, Wittinghofer C, Armbrecht AS, Stahl J. Detection of deception: Event-related potential markers of attention and cognitive control during intentional false responses. Psychophysiology. 2018;55(6). e13047. [PubMed ID: 29226961]. https://doi.org/10.1111/psyp.13047.
-
9.
Stewart SLK, Wright C, Atherton C. Deception Detection and Truth Detection Are Dependent on Different Cognitive and Emotional Traits: An Investigation of Emotional Intelligence, Theory of Mind, and Attention. Pers Soc Psychol Bull. 2019;45(5):794-807. [PubMed ID: 30264653]. https://doi.org/10.1177/0146167218796795.
-
10.
Suchotzki K, Verschuere B, Van Bockstaele B, Ben-Shakhar G, Crombez G. Lying takes time: A meta-analysis on reaction time measures of deception. Psychol Bull. 2017;143(4):428-53. [PubMed ID: 28182460]. https://doi.org/10.1037/bul0000087.
-
11.
Park KK, Suk HW, Hwang H, Lee JH. A functional analysis of deception detection of a mock crime using infrared thermal imaging and the Concealed Information Test. Front Hum Neurosci. 2013;7:70. [PubMed ID: 23470924]. [PubMed Central ID: PMC3590493]. https://doi.org/10.3389/fnhum.2013.00070.
-
12.
Wu Z, Singh B, Davis L, Subrahmanian V, editors. Deception detection in videos. Proceedings of the AAAI conference on artificial intelligence. 2018; Louisiana, USA. California, USA: AAAI Press; 2018. p. 1695-702.
-
13.
Ondras J, Gunes H, editors. Detecting Deception and Suspicion in Dyadic Game Interactions. 20th ACM International Conference on Multimodal Interaction. 2018; Colorado, USA. Association for Computing Machinery; 2018. p. 200-9.
-
14.
Raskin DC. Polygraph techniques for the detection of deception. Psychological methods in criminal investigation and evidence. Berlin, Germany: Springer; 1989. p. 247-96.
-
15.
Phan KL, Magalhaes A, Ziemlewicz TJ, Fitzgerald DA, Green C, Smith W. Neural correlates of telling lies: a functional magnetic resonance imaging study at 4 Tesla. Acad Radiol. 2005;12(2):164-72. [PubMed ID: 15721593]. https://doi.org/10.1016/j.acra.2004.11.023.
-
16.
Monteleone GT, Phan KL, Nusbaum HC, Fitzgerald D, Irick JS, Fienberg SE, et al. Detection of deception using fMRI: better than chance, but well below perfection. Soc Neurosci. 2009;4(6):528-38. [PubMed ID: 18633832]. https://doi.org/10.1080/17470910801903530.
-
17.
Lee TM, Liu HL, Tan LH, Chan CC, Mahankali S, Feng CM, et al. Lie detection by functional magnetic resonance imaging. Hum Brain Mapp. 2002;15(3):157-64. [PubMed ID: 11835606]. [PubMed Central ID: PMC6872015]. https://doi.org/10.1002/hbm.10020.
-
18.
Al-Hamouri FA. Hemispheric dominance for deception: A dual-task performance study. Int J Humanit Soc Sci. 2012;2:168-71.
-
19.
Ganis G, Kosslyn SM, Stose S, Thompson WL, Yurgelun-Todd DA. Neural correlates of different types of deception: an fMRI investigation. Cereb Cortex. 2003;13(8):830-6. [PubMed ID: 12853369]. https://doi.org/10.1093/cercor/13.8.830.
-
20.
Langleben DD, Loughead JW, Bilker WB, Ruparel K, Childress AR, Busch SI, et al. Telling truth from lie in individual subjects with fast event-related fMRI. Hum Brain Mapp. 2005;26(4):262-72. [PubMed ID: 16161128]. [PubMed Central ID: PMC6871667]. https://doi.org/10.1002/hbm.20191.
-
21.
Abe N, Suzuki M, Mori E, Itoh M, Fujii T. Deceiving others: distinct neural responses of the prefrontal cortex and amygdala in simple fabrication and deception with social interactions. J Cogn Neurosci. 2007;19(2):287-95. [PubMed ID: 17280517]. https://doi.org/10.1162/jocn.2007.19.2.287.
-
22.
Ganis G. Detecting Deception and Concealed Information With Neuroimaging. In: Rosenfeld J, editor. Detecting Concealed Information and Deception. Massachusetts, USA: Academic Press; 2018. p. 145-66. https://doi.org/10.1016/b978-0-12-812729-2.00007-0.
-
23.
Spence SA, Hunter MD, Farrow TF, Green RD, Leung DH, Hughes CJ, et al. A cognitive neurobiological account of deception: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2004;359(1451):1755-62. [PubMed ID: 15590616]. [PubMed Central ID: PMC1693447]. https://doi.org/10.1098/rstb.2004.1555.
-
24.
Takata N, Sugiura Y, Yoshida K, Koizumi M, Hiroshi N, Honda K, et al. Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia. 2018;66(9):2013-23. [PubMed ID: 29845643]. https://doi.org/10.1002/glia.23454.
-
25.
Westen D, Shedler J. A prototype matching approach to diagnosing personality disorders: toward DSM-V. J Pers Disord. 2000;14(2):109-26. [PubMed ID: 10897462]. https://doi.org/10.1521/pedi.2000.14.2.109.
-
26.
Bryden MP. Measuring handedness with questionnaires. Neuropsychologia. 1977;15(4-5):617-24. [PubMed ID: 896019]. https://doi.org/10.1016/0028-3932(77)90067-7.
-
27.
Ben-Shakhar G, Elaad E. The Guilty Knowledge Test (GKT) as an application of psychophysiology: Future prospects and obstacles. In: Kleiner M, editor. Handbook of polygraph testing. Massachusetts, USA: Academic Press; 2002. p. 87-102.
-
28.
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-90. [PubMed ID: 21979382]. https://doi.org/10.1016/j.neuroimage.2011.09.015.
-
29.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825-41. [PubMed ID: 12377157]. https://doi.org/10.1016/s1053-8119(02)91132-8.
-
30.
Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143-56. [PubMed ID: 11516708]. https://doi.org/10.1016/s1361-8415(01)00036-6.
-
31.
Jenkinson M, Pechaud M, Smith S, editors. BET2: MR-based estimation of brain, skull and scalp surfaces. Eleventh annual meeting of the organization for human brain mapping. 2005; Toronto, Canada. MonetLab; 2005. 167 p.
-
32.
Kozel FA, Johnson KA, Mu Q, Grenesko EL, Laken SJ, George MS. Detecting deception using functional magnetic resonance imaging. Biol Psychiatry. 2005;58(8):605-13. [PubMed ID: 16185668]. https://doi.org/10.1016/j.biopsych.2005.07.040.
-
33.
Nunez JM, Casey BJ, Egner T, Hare T, Hirsch J. Intentional false responding shares neural substrates with response conflict and cognitive control. Neuroimage. 2005;25(1):267-77. [PubMed ID: 15734361]. https://doi.org/10.1016/j.neuroimage.2004.10.041.
-
34.
Hu X, Chen H, Fu G. A repeated lie becomes a truth? The effect of intentional control and training on deception. Front Psychol. 2012;3:488. [PubMed ID: 23162520]. [PubMed Central ID: PMC3495335]. https://doi.org/10.3389/fpsyg.2012.00488.
-
35.
Abe N. How the brain shapes deception: an integrated review of the literature. Neuroscientist. 2011;17(5):560-74. [PubMed ID: 21454323]. https://doi.org/10.1177/1073858410393359.
-
36.
Cui F, Wu S, Wu H, Wang C, Jiao C, Luo Y. Altruistic and self-serving goals modulate behavioral and neural responses in deception. Soc Cogn Affect Neurosci. 2018;13(1):63-71. [PubMed ID: 29149322]. [PubMed Central ID: PMC5793826]. https://doi.org/10.1093/scan/nsx138.
-
37.
Gao M, Yang X, Shi J, Lin Y, Chen S. Does Gender Make a Difference in Deception? The Effect of Transcranial Direct Current Stimulation Over Dorsolateral Prefrontal Cortex. Front Psychol. 2018;9:1321. [PubMed ID: 30177894]. [PubMed Central ID: PMC6109782]. https://doi.org/10.3389/fpsyg.2018.01321.
-
38.
Sip KE, Skewes JC, Marchant JL, McGregor WB, Roepstorff A, Frith CD. What if I Get Busted? Deception, Choice, and Decision-Making in Social Interaction. Front Neurosci. 2012;6:58. [PubMed ID: 22529772]. [PubMed Central ID: PMC3328780]. https://doi.org/10.3389/fnins.2012.00058.
-
39.
Abe N, Suzuki M, Tsukiura T, Mori E, Yamaguchi K, Itoh M, et al. Dissociable roles of prefrontal and anterior cingulate cortices in deception. Cereb Cortex. 2006;16(2):192-9. [PubMed ID: 15858160]. https://doi.org/10.1093/cercor/bhi097.
-
40.
Ganis G, Rosenfeld JP, Meixner J, Kievit RA, Schendan HE. Lying in the scanner: covert countermeasures disrupt deception detection by functional magnetic resonance imaging. Neuroimage. 2011;55(1):312-9. [PubMed ID: 21111834]. https://doi.org/10.1016/j.neuroimage.2010.11.025.
-
41.
Chun JW, Park HJ, Kim DJ, Kim E, Kim JJ. Contribution of fronto-striatal regions to emotional valence and repetition under cognitive conflict. Brain Res. 2017;1666:48-57. [PubMed ID: 28477862]. https://doi.org/10.1016/j.brainres.2017.04.018.
-
42.
Baldwin PA, Whitford TJ, Grisham JR. Emotion Sensitivity of the Error-Related Negativity in Hoarding Individuals. J Psychopathol Behav Assess. 2019;41(4):589-97. https://doi.org/10.1007/s10862-018-09716-9.
-
43.
Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17(4):1820-9. [PubMed ID: 12498755]. https://doi.org/10.1006/nimg.2002.1326.
-
44.
Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539-46. [PubMed ID: 15556023]. https://doi.org/10.1016/j.tics.2004.10.003.
-
45.
Sripada CS, Gonzalez R, Phan KL, Liberzon I. The neural correlates of intertemporal decision-making: contributions of subjective value, stimulus type, and trait impulsivity. Hum Brain Mapp. 2011;32(10):1637-48. [PubMed ID: 20886577]. [PubMed Central ID: PMC6870159]. https://doi.org/10.1002/hbm.21136.
-
46.
Venkatraman V, Rosati AG, Taren AA, Huettel SA. Resolving response, decision, and strategic control: evidence for a functional topography in dorsomedial prefrontal cortex. J Neurosci. 2009;29(42):13158-64. [PubMed ID: 19846703]. [PubMed Central ID: PMC2801415]. https://doi.org/10.1523/JNEUROSCI.2708-09.2009.
-
47.
Mitchell DG, Luo Q, Avny SB, Kasprzycki T, Gupta K, Chen G, et al. Adapting to dynamic stimulus-response values: differential contributions of inferior frontal, dorsomedial, and dorsolateral regions of prefrontal cortex to decision making. J Neurosci. 2009;29(35):10827-34. [PubMed ID: 19726640]. [PubMed Central ID: PMC2774778]. https://doi.org/10.1523/JNEUROSCI.0963-09.2009.
-
48.
Piva M, Velnoskey K, Jia R, Nair A, Levy I, Chang SW. The dorsomedial prefrontal cortex computes task-invariant relative subjective value for self and other. Elife. 2019;8. [PubMed ID: 31192786]. [PubMed Central ID: PMC6565363]. https://doi.org/10.7554/eLife.44939.
-
49.
Oldrati V, Patricelli J, Colombo B, Antonietti A. The role of dorsolateral prefrontal cortex in inhibition mechanism: A study on cognitive reflection test and similar tasks through neuromodulation. Neuropsychologia. 2016;91:499-508. [PubMed ID: 27647553]. https://doi.org/10.1016/j.neuropsychologia.2016.09.010.
-
50.
Mandal AS, Fama ME, Skipper-Kallal LM, DeMarco AT, Lacey EH, Turkeltaub PE. Brain structures and cognitive abilities important for the self-monitoring of speech errors. Neurobiol Lang (Camb). 2020;1(3):319-38. [PubMed ID: 34676371]. [PubMed Central ID: PMC8528269]. https://doi.org/10.1162/nol_a_00015.
-
51.
Manna A, Raffone A, Perrucci MG, Nardo D, Ferretti A, Tartaro A, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res Bull. 2010;82(1-2):46-56. [PubMed ID: 20223285]. https://doi.org/10.1016/j.brainresbull.2010.03.001.
-
52.
Kahane G. Sidetracked by trolleys: Why sacrificial moral dilemmas tell us little (or nothing) about utilitarian judgment. Soc Neurosci. 2015;10(5):551-60. [PubMed ID: 25791902]. [PubMed Central ID: PMC4642180]. https://doi.org/10.1080/17470919.2015.1023400.
-
53.
Turnbull A, Wang HT, Murphy C, Ho NSP, Wang X, Sormaz M, et al. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nat Commun. 2019;10(1):3816. [PubMed ID: 31444333]. [PubMed Central ID: PMC6707151]. https://doi.org/10.1038/s41467-019-11764-y.
-
54.
Knoch D, Fehr E. Resisting the power of temptations: the right prefrontal cortex and self-control. Ann N Y Acad Sci. 2007;1104:123-34. [PubMed ID: 17344543]. https://doi.org/10.1196/annals.1390.004.
-
55.
Xu T, Sirois FM, Zhang L, Yu Z, Feng T. Neural basis responsible for self-control association with procrastination: Right MFC and bilateral OFC functional connectivity with left dlPFC. J Res Pers. 2021;91:104064. https://doi.org/10.1016/j.jrp.2021.104064.
-
56.
Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. [PubMed ID: 18939997]. [PubMed Central ID: PMC2588614]. https://doi.org/10.1186/1471-2202-9-102.
-
57.
Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313-9. [PubMed ID: 20056157]. [PubMed Central ID: PMC2845804]. https://doi.org/10.1016/j.neuroimage.2009.12.109.
-
58.
Andrew D; Muhlert; Nils; Boy; Frederick; Lawrence. Risk Taking, Response Inhibition and the Right Inferior Frontal Gyrus. J Neurol Neurosurg Psychiatry. 2015;86(9):e3.33-e3. https://doi.org/10.1136/jnnp-2015-311750.39.
-
59.
Pan PL, Song W, Shang HF. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson's disease. Eur J Neurol. 2012;19(2):199-206. [PubMed ID: 21762435]. https://doi.org/10.1111/j.1468-1331.2011.03474.x.
-
60.
Vartanian O, Kwantes PJ, Mandel DR, Bouak F, Nakashima A, Smith I, et al. Right inferior frontal gyrus activation as a neural marker of successful lying. Front Hum Neurosci. 2013;7:616. [PubMed ID: 24106468]. [PubMed Central ID: PMC3789213]. https://doi.org/10.3389/fnhum.2013.00616.
.jpg)