Abstract
Background:
Anxiety and depression are common nonmotor symptoms of Parkinson’s disease (PD).Objectives:
We investigated whether Bisphenol A (BPA) is capable of inducing anxiety/depressive-like behaviors and neurotransmitter alterations that are similar to those observed in PD.Materials and Methods:
To test this hypothesis, we used a zebrafish animal model and conducted behavioral and histological assays.Results:
BPA produced anxiety/depression-like behavioral signs for 14 days following administration. Altered behavioral responses were accompanied by reductions of striatal dopamine transporter, and decrease in hippocampal 5-HT content.Conclusions:
These results suggest that the nigrostriatal pathway might play a role in the etiology of anxiety/depression. Furthermore, dopamine transporter function, in particular, might play a critical role in the pathophysiology of anxiety/depression.Keywords
1. Background
Parkinson's disease (PD) is clinically based on the presence of a set of cardinal motor signs including rigidity, bradykinesia, resting tremor and postural reflex disturbances. However, in many cases, nonmotor alterations (anxiety, depression, sleep, gastrointestinal and cognitive functions) precede the classical motor symptoms in PD, and the management of these nonmotor symptoms is clinically challenging (1-3).I have added this reference to the reference section. Anxiety/depression in PD is thought to result from factors such as disappearance of dopaminergic, noradrenergic and serotoninergic neurons, as well as from psychogenic reactions associated with the onset of disease (4). A pattern of regional neurodegeneration that varies considerably depending upon the affected neuronal population may explain different symptoms observed in such patients. In fact, differential mechanisms of neuronal vulnerability within the substantia nigra pars compacta suggests that factors other than location contribute to the susceptibility of these neurons (3).
Several lines of evidence have shown that 5-HT1A receptors play an important role in controlling motor functions and ameliorate various extrapyramidal disorders such as PD (5-9) and L-DOPA-induced motor disabilities (10-13). In addition, 5-HT1A receptors are implicated in the pathogenesis and treatment of mood disorders (anxiety/depression) (8). Loss of 5-HT neurons in the raphe nuclei has been reported (14). Therefore, 5-HT content and the density of 5-HT transporters (a marker for 5-HT nerve terminals) in the forebrain regions (i.e., striatum and neocortex) are reduced in patients with PD. Postsynaptic 5-HT1A and 5-HT2A receptors are upregulated in response to the functional deficits of 5-HT neurons (14). Furthermore, recent clinical studies have demonstrated that patients with Parkinson and depression show further pronounced dysfunction of 5-HT neurons as compared to those without depression (15). Thus, there is a close association between dysfunction of the serotonergic system and PD (Figure 1).
Schematic View of the Neural Network Regulating Motor and Behavioral Functions in Parkinson’s Disease
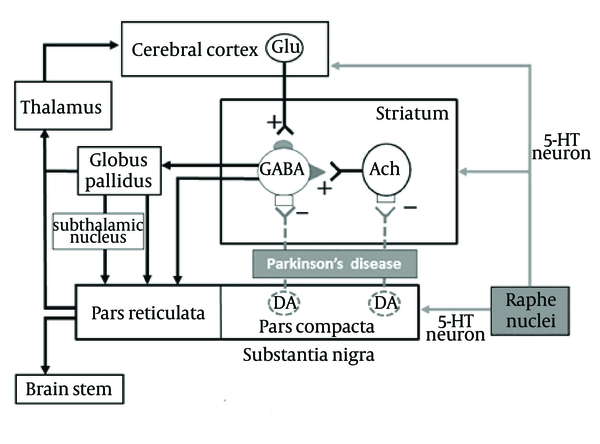
Although the monoamines, specifically serotonin and norepinephrine have been targets for the development of novel antidepressants. Some of the effectiveness of the classical SSRI’s is mediated via the dopaminergic system; Wellbutrin ® (bupropion) is a classic example (16). Dopaminergic antidepressant drugs, such as specific dopamine reuptake inhibitors like amineptine, the presynaptic dopamine antagonist amisulpride, and the D2/D3 agonist roxindole and pramipexole, all cause rapid relief of symptoms. Low levels of dopamine metabolites have been detected in CSF of patients with depression (17). Reports of low levels of dopamine metabolite dihydroxyphenylacetic acid in CSF of patients with depression (18) and in the basal ganglia of postmortem brains of patients with depression who committed suicide (19), lend additional support to a theory of dopamine abnormality in depression. The aim of our study was to investigate the dopaminergic/serotonergic mechanism-of-action to induce anxiety/depression.
2. Objectives
Anxiety and depression are common nonmotor symptoms of Parkinson’s disease (PD). We investigated whether Bisphenol A (BPA) is capable of inducing anxiety/depressive-like behaviors and neurotransmitter alterations that are similar to those observed in PD. To test this hypothesis, we used a zebrafish animal model and conducted behavioral and histological assays. BPA produced anxiety/depression-like behavioral symptoms for 14 days following administration.
3. Patients and Methods
3.1. Animals and Housing
Adult Zebrafish (> 90 days; 70 male) were obtained from a local commercial distributer (Petsmart; Destin, FL, USA). The fish were housed in 1 L tanks (Thoren Aquatics: recirculating high-density rack system specifically designed for zebrafish) in groups of five fish per tank. The water was filtered using mechanical (sponge), chemical (activated carbon), and biological filtration units. The tanks were filled with deionized water treated with concentrated conditioner (Prime Freshwater®,USA ). Water temperature was maintained at 25-27°C. Lights were on a timer and turned on from 9:00 AM to 7:00 PM every day. All fish were fed a mixture of ground flake food (Tetramin Tropical Flakes; Tetra USA, Blacksburg, VA). All fish were maintained and procedures performed in accordance with the Institutional Animal Care and Use Committee of Troy University, Troy, AL, USA.
3.2. Treatments
Ten adult male zebrafish were randomly assigned to a treatment group. The zebrafish were separately exposed to 0, 5, 10, 15, 20, 30, 50 mg/L of BPA (20-22) using 0.05% ethanol (vehicle). The control group was exposed to 0.05% ethanol (vehicle)/water. Groups were exposed to the above treatments for one hour a day (in a separate beaker) for three weeks. Exposure times for the 30 and 50 mg/L BPA were 40 and 10 minutes, respectively; this was the maximal tolerated exposure period.
3.3. Behavioral Analysis
A novel tank diving test was conducted employing the methodology by Levin et al (23). All the behavioral tests were video-recorded by iPhone. Briefly, the fish were placed individually in a 5-Liter tank (approximately 20 cm H x 30 cm TW) maximally filled with aquarium treated tap water. The tank was divided into two equal horizontal halves, marked by a Sharpie® on the outside of the tank. The tank rested on a stable, level surface, and all environmental distractions were kept to a minimum. An iPhone® was placed approximately 30 centimeter in front of the novel tank to ensure that the novel tank was within the iPhone cameras’ vision range. A standard letter size (22 cm x 28 cm) sheet of paper was placed behind and on the sides of the novel tank to ensure a uniform background for video analysis. Fish were transported from their home tank to the novel tank with care to reduce net-stress. Once placed in the novel tank, zebrafish movement was recorded for 5 minutes. Tracker 4.81 (24) was used to analyze all of the coordinates of the zebrafish movements. A coordinate system was built with the horizontal line marked in the middle of the tank as the x axis, and the left side of the tank as the y axis. The step size of the frames was set as 30. A zebrafish point mass was created and auto-tracked (a manual tracking option can be used for corrections) by this software. The time (t) and related coordinates (x, y) of the point mass (zebrafish) were recorded every one second by Tracker 4.81. Three hundred points were recorded for each 5 minutes of video. All of the coordinate data was calculated using Excel (Microsoft, USA). The erratic movements of the fish were considered as the sum of distance between the two points (fish coordinate locations). The time in the upper half of the tank was calculated from the number of positive “y” coordinates. The transitions to upper half were counted as the number of negative “yn+1 / yn”. The freezing bouts times were considered as the distance change between the two points less than 0.5. Examples of the functions used in Excel are shown below:
1) Distance (Dn = 4) = SQRT ((B4-B3) ^2 + (C4-C3) ^2)
2) Erratic movements = Sum (D4: D303)
3) Time in upper half = COUNTIF (C3:C303,"> 0")
4) yn + 1/yn = C4/C3
5) Transitions to upper half = COUNTIF (G4:G303, "< 0")
6) Dn+1-Dn = D5-D4
7) ABS (Dn + 1-Dn) = ABS (D5-D4)
8) Freezing bout = COUNTIF (K4:K303,"< 0.5")
3.4. Immunohistochemistry
Immunohistochemical staining within the subpallium (striatum) and hippocampus of the zebrafish brain was performed to detect changes in neuronal numbers following BPA exposure. Brains were isolated and fixed in 4% paraformaldehyde overnight at 4°C. After rinsing in phosphate-buffered saline (PBS) (pH 7.4), the brains were incubated in 30% sucrose. Sections were cut on a cryostat at 10 µm. Antibody characterization was via monoclonal antibodies against DAT and 5-HT (Chemicon Int., Temecula, CA, USA). Differences in immunoreactive DAT and 5-HT were tested using an established immunohistochemistry procedure in our laboratory. Briefly, the brain was prepared by making coronal slices, with a razor blade, at the caudal-most region of the olfactory bulbs and the rostral most portion of the brain. The brain was then rapidly frozen on a cryostat stage. The brain was then prepared for cutting using OTC (Tissue-Tek, Miles Inc., Elkhart, IN) over the outer surface of the brain. Two consecutive 10µm sections were placed on two separate sets of 0.5% gelatin-coated slides, one control and one treated. This process was repeated every 25 µm. For each case, the matched control brain and treated brain were placed adjacent to one another on each of the slides. This ensured that sections from the treated and matched vehicle control brains were always exposed to identical tissue processing conditions. Sections were counter-balanced between cases to prevent possible position effects. In addition, each set of slides from a given case included an additional slide used as an omission control for nonspecific staining. After cutting the tissue, a circle was made around each section using a “PAP” pen, hydrophobic marker, to facilitate the containment of incubation solutions placed on the sections. Brain tissue was processed by the avidin-biotin-peroxidase complex (ABC) method. Slides were gently rinsed with PBS and placed in Coplin jars filled with PBS. Slides were then dried using Kimwipes and incubated for 20 minutes in a 10% blocking serum (10% normal goat serum (Vector Labs, Burlingame, CA) in PBS with 15% Triton-X-100) in a humidity chamber. Slides were then dried again and incubated in well characterized, commercially available primary antibody to DAT and 5-HT. Primary antibodies were diluted with a solution of 1% goat serum in PBS with 0.15% Triton-X-100 to 600:1 for 18 hours in a humidity chamber. Omission control sections for the case were processed in identical solutions but without primary antibody exposure. At the end of the primary antibody incubation, slides were gently rinsed and placed in Coplin jars for 3 X 5 minute wash baths (PBS). The tissue was then incubated for 45 minutes in a biotinylated secondary antibody solution, biotinylated goat antirabbit secondary antibody solution (Vector Labs, Burlingame, CA) in 1% diluent with 0.15% Trition-X-100 in an incubation chamber. Following the biotinylated antibody incubation, the slides were then rinsed (3 X 5 minute wash baths (PBS)), then incubated in 3% hydrogen peroxide in PBS for 10 minutes (to quench endogenous peroxidase activity), then rinsed (3 X 5 minute wash baths (PBS)). The sections were then incubated for 45 minutes in an avidin-biotin peroxidase complex solution (ABC elite kit, Vector laboratories). At the end of the ABC incubation, the slides were rinsed again (3 X 5 minute wash baths (PBS)). Immunoreactivity was revealed by incubation in diaminobenzidine (DAB) solution (0.05% diaminobenzidine and 66 µL of 30% hydrogen peroxide in PBS). Sections were incubated in DAB for 5 minutes. Following DAB incubation, slides were rinsed (3 X 5 minute wash baths (PBS)). Sections were then dehydrated through a series of ethanol baths (70%, 80%, 95%, and 100%), cleared in xylene, and coverslipped with Permount®.
3.5. Image Analysis
The digital images captured from each section were then analyzed using the “Histogram” tool of Adobe Photoshop© software (Adobe Systems Incorporated, USA). This computerized methodology eliminates any observer bias regarding immunostaining counts. For any selected area of pixels from a digital photograph, this function can determine the mean gray scale value (0-255, where 0 is black and 255 is white) and the number of pixels that are darker than a specified gray scale value. All images were converted to gray scale and a mean minimum gray scale value was determined for immunolabeled varicosities. This gray scale value was determined from all vehicle control brains. All pixels darker than this minimum value would be subsequently counted as positive immunostaining. The mean minimum gray scale value was determined by finding the mean gray scale value of five immunolabeled varicosities per field (loci), judged as the minimum acceptable for measurement. The grand section mean across the four fields of each section was then determined. Then a grand mean for the brain was determined across each of the grand section means. The minimum acceptable gray scale value, calculated from all the vehicle control brains, was then used to make pixel counts on both the vehicle control and treated brains. The number of pixels that had a value less than, ie, darker than, the minimum acceptable gray scale value was recorded for each field within a brain section. The count for each field was used to calculate a mean subpallium (striatum) pixel count of immunopositive labeling for each section. The mean pixel count for each treated section on a single slide was compared with its adjacent, matched vehicle-treated section. The mean of these differences was then calculated across slides for a given case (case = one treated brain and matched vehicle control brain). These mean differences in pixel counts were tested for their difference from zero.
3.6. Data Analysis
Using SPSS 10.0 Software (SPSS Inc., Chicago, IL) an ANOVA (analysis of variance) was performed for each of the BPA concentrations for both the behavioral and immunohistochemistry data sets. Data comparisons were performed with a Tukey HSD post hoc test. Data was considered significantly different at α < 0.05.
4. Results
4.1. Behavioral Analysis
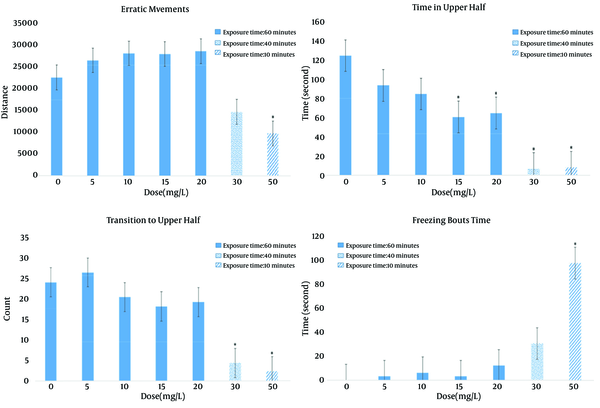
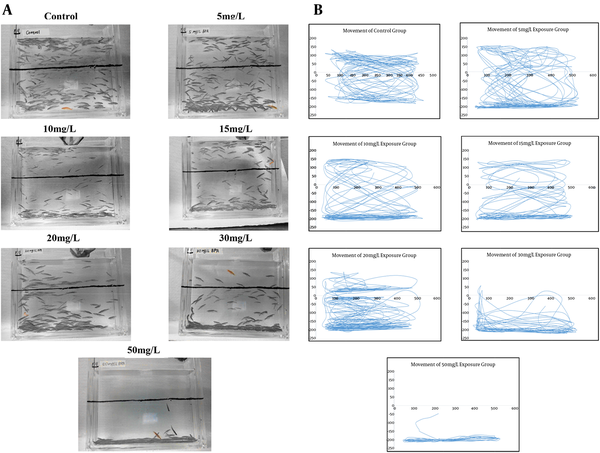
Regarding the behavioral results using the Novel Tank Diving Test, Figure 2 shows the number of erratic movements, transitions to the upper half of the tank, amount of freezing behavior, and total time spent in the upper half of the tank of experimental fish compared to control. Movement was impacted in a dose-dependent manner. In particular, significant deficits in movement were observed with 30 and 50 mg/L of BPA. No significant erratic movements and freezing bout time were found in lower dosage groups compared to the control group; while significant changes were found in the 50 mg/L treatment group (F = 10.809, P = 0.001). Time in the upper half of the tank was significantly decreased in the 15 mg/L treatment group (P = 0.005), 20 mg/L group (P = 0.008), 30 mg/L group (P < 0.001), and 50 mg/L (P < 0.001). There was a significant decrease in the total number of transitions to upper half in the 30 mg/L (P = 0.021) and 50 mg/L (P = 0.005) dosage group. Using tracking techniques, as shown in Figure 3 (A, B), these movement deficits can be better appreciated.
Novel Tank Diving Test Spontaneous Movement Analysis
Representative Filter Images and Tracings of Five Minutes Swimming Tracks After Exposure to Bisphenol A
4.2. Immunohistochemistry Analysis
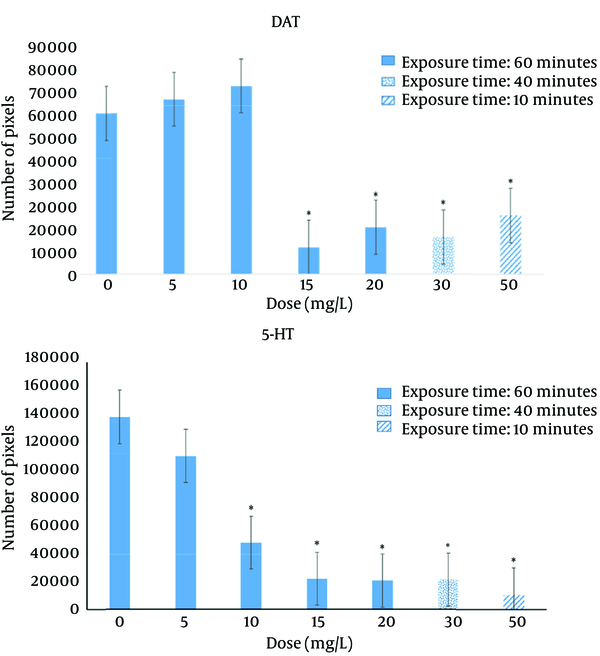
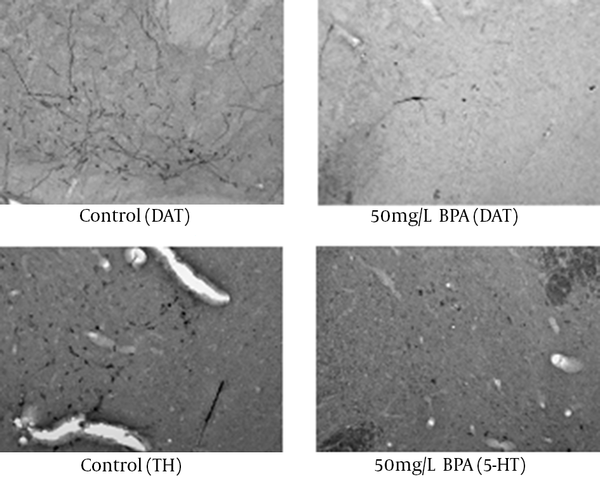
Significant decreases in DAT and 5-HT were found following BPA exposure regarding immunohistochemistry. For DAT (Figure 4), compared to the control group, significant reductions were found in 15 mg/L (P = 0.002), 20 mg/L (P = 0.011), 30 mg/L (P = 0.005), and 50 mg/L (P = 0.033) groups. For 5-HT, compared to the control group, significant decreases were found in all the dosage groups (P < 0.001). Representative examples of the DAT and 5-HT immunohistochemistry are shown in Figure 5. Figure 6 illustrates the anatomical areas of immunohistological staining.
Changes in DAT and 5-HT immunoreactivity
Representative Examples of Immunohistological Staining for DAT and 5-HT Immunoreactivity
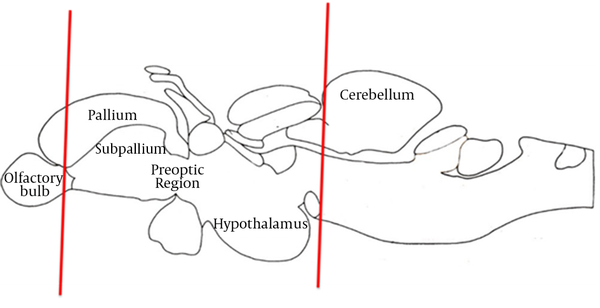
Anatomical Regions of Tissue Acquisition for Immunohistochemical Analysis in the Zebrafish Brain
5. Discussion
Activation of 5-HT1A receptors is involved in the treatment of anxiety and depression. However, designing compounds, which possess combined activities for both 5-HT1A and dopamine receptors, seems to be a promising approach to the treatment. Therefore, zebrafish dopaminergic population corresponding to the mammalian substantia nigra is still a matter of debate. The strongest candidate now is the posterior tuberculum, based on immunohistochemical and tracer studies. The posterior tuberculum sends connections to the subpallium, which is the putative zebrafish striatum. However, the corticostriatal pathway regulated by dopamine is poorly characterized in fish. Better characterization of these pathways is needed prior to the zebrafish model for PD to become fully accepted and widely used. Future studies based on zebrafish models, together with additional research using other species, would be instrumental in the development of effective drugs for PD as well as for other neurodegenerative diseases. The findings of the present investigation suggest that exposure to BPA at the doses, exposure intervals, and conditions tested have a capacity to alter the normal functioning of the nigrostriatal pathway. Overall, these results suggest that the nigrostriatal pathway plays a role in anxiety and depression. Decreased DAT availability is not the only factor associated with the development of anxiety/depression-like symptoms in PD. Other neurotransmitter systems, including norepinephrine, serotonin and acetylcholine may also play a role in anxiety and depression in PD. All these neurotransmitter systems significantly interact in a complex pattern with the dopaminergic system and are likely to be affected in PD. In this respect, caution is required in the interpretation of our results. Nevertheless, it is becoming increasingly apparent that dopamine plays a more prominent role than once thought regarding the development of anxiety/depression-like symptoms.
Acknowledgements
References
-
1.
Meireles J, Massano J. Cognitive impairment and dementia in Parkinson's disease: clinical features, diagnosis, and management. Front Neurol. 2012;3:88. [PubMed ID: 22654785]. https://doi.org/10.3389/fneur.2012.00088.
-
2.
Ohno Y. Mapping the progress of Alzheimer's and Parkinson's disease. In: Mizuno Y, Fisher A, Hanin A, editors. Mapping the progress of Alzheimer's and Parkinson's disease. New York: Academic/Plenum; 2002. p. 423-8. https://doi.org/10.1007/978-0-306-47593-1_72.
-
3.
Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363(9423):1783-93. [PubMed ID: 15172778]. https://doi.org/10.1016/s0140-6736(04)16305-8.
-
4.
Richard IH, Schiffer RB, Kurlan R. Anxiety and Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1996;8(4):383-92. [PubMed ID: 9116473].
-
5.
Ishibashi T, Ohno Y. Antiparkinsonian actions of a selective 5-HT 1A agonist, tandospirone, in rats. Biogenic Amines, 18. 2004;3(6):329-38. https://doi.org/10.1163/1569391041501924.
-
6.
Matsubara K, Shimizu K, Suno M, Ogawa K, Awaya T, Yamada T, et al. Tandospirone, a 5-HT1A agonist, ameliorates movement disorder via non-dopaminergic systems in rats with unilateral 6-hydroxydopamine-generated lesions. Brain Res. 2006;1112(1):126-33. [PubMed ID: 16884702]. https://doi.org/10.1016/j.brainres.2006.07.003.
-
7.
Mignon L, Wolf WA. Postsynaptic 5-HT(1A) receptors mediate an increase in locomotor activity in the monoamine-depleted rat. Psychopharmacology (Berl). 2002;163(1):85-94. [PubMed ID: 12185404]. https://doi.org/10.1007/s00213-002-1121-3.
-
8.
Ohno Y. New insight into the therapeutic role of 5-HT1A receptors in central nervous system disorders. Cent Nerv Syst Agents Med Chem. 2010;10(2):148-57. [PubMed ID: 20518729].
-
9.
Ohno Y, Shimizu S, Imaki J, Ishihara S, Sofue N, Sasa M, et al. Evaluation of the antibradykinetic actions of 5-HT1A agonists using the mouse pole test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1302-7. [PubMed ID: 18495311]. https://doi.org/10.1016/j.pnpbp.2008.04.005.
-
10.
Bishop C, Krolewski DM, Eskow KL, Barnum CJ, Dupre KB, Deak T, et al. Contribution of the striatum to the effects of 5-HT1A receptor stimulation in L-DOPA-treated hemiparkinsonian rats. J Neurosci Res. 2009;87(7):1645-58. [PubMed ID: 19115412]. https://doi.org/10.1002/jnr.21978.
-
11.
Cheshire PA, Williams DR. Serotonergic involvement in levodopa-induced dyskinesias in Parkinson's disease. J Clin Neurosci. 2012;19(3):343-8. [PubMed ID: 22249009]. https://doi.org/10.1016/j.jocn.2011.09.008.
-
12.
Dupre KB, Eskow KL, Steiniger A, Klioueva A, Negron GE, Lormand L, et al. Effects of coincident 5-HT1A receptor stimulation and NMDA receptor antagonism on L-DOPA-induced dyskinesia and rotational behaviors in the hemi-parkinsonian rat. Psychopharmacology (Berl). 2008;199(1):99-108. [PubMed ID: 18545986]. https://doi.org/10.1007/s00213-008-1135-6.
-
13.
Tomiyama M, Kimura T, Maeda T, Kannari K, Matsunaga M, Baba M. A serotonin 5-HT1A receptor agonist prevents behavioral sensitization to L-DOPA in a rodent model of Parkinson's disease. Neurosci Res. 2005;52(2):185-94. [PubMed ID: 15893579]. https://doi.org/10.1016/j.neures.2005.03.004.
-
14.
Chen CP, Alder JT, Bray L, Kingsbury AE, Francis PT, Foster OJ. Post-synaptic 5-HT1A and 5-HT2A receptors are increased in Parkinson's disease neocortex. Ann N Y Acad Sci. 1998;861:288-9. [PubMed ID: 9928295].
-
15.
Huot P, Johnston TH, Visanji NP, Darr T, Pires D, Hazrati LN, et al. Increased levels of 5-HT1A receptor binding in ventral visual pathways in Parkinson's disease. Mov Disord. 2012;27(6):735-42. [PubMed ID: 22419526]. https://doi.org/10.1002/mds.24964.
-
16.
Smith GS, Dewey SL, Brodie JD, Logan J, Vitkun SA, Simkowitz P, et al. Serotonergic modulation of dopamine measured with [11C]raclopride and PET in normal human subjects. Am J Psychiatry. 1997;154(4):490-6. [PubMed ID: 9090335].
-
17.
Willner P, D'Aquila PS, Coventry T, Brain P. Loss of social status: preliminary evaluation of a novel animal model of depression. J Psychopharmacol. 1995;9(3):207-13. [PubMed ID: 22297759]. https://doi.org/10.1177/026988119500900302.
-
18.
Roy A, Pickar D, Linnoila M, Doran AR, Ninan P, Paul SM. Cerebrospinal fluid monoamine and monoamine metabolite concentrations in melancholia. Psychiatry Res. 1985;15(4):281-92. [PubMed ID: 2415996].
-
19.
Bowden C, Cheetham SC, Lowther S, Katona CL, Crompton MR, Horton RW. Reduced dopamine turnover in the basal ganglia of depressed suicides. Brain Res. 1997;769(1):135-40. [PubMed ID: 9374281].
-
20.
Van den Belt K, Verheyen R, Witters H. Comparison of vitellogenin responses in zebrafish and rainbow trout following exposure to environmental estrogens. Ecotoxicol Environ Saf. 2003;56(2):271-81. [PubMed ID: 12927559].
-
21.
Villeneuve DL, Garcia-Reyero N, Escalon BL, Jensen KM, Cavallin JE, Makynen EA, et al. Ecotoxicogenomics to support ecological risk assessment: a case study with bisphenol A in fish. Environ Sci Technol. 2012;46(1):51-9. [PubMed ID: 21786754]. https://doi.org/10.1021/es201150a.
-
22.
vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24(2):131-8. [PubMed ID: 17768031]. https://doi.org/10.1016/j.reprotox.2007.07.005.
-
23.
Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90(1):54-8. [PubMed ID: 17049956]. https://doi.org/10.1016/j.physbeh.2006.08.026.
-
24.
Tracker. 2013, [updated 2014]. Available from: http://www.cabrillo.edu/~dbrown/tracker/.