Abstract
Background:
The exact pathogenesis of epilepsy is not clear well. Recent studies showed the possible involvement of transient receptor potential vanilloid type 1 (TRPV1) receptors in this disease.Objectives:
The aim of this study was to measure the expression of TRPV1 receptors in hippocampus following pentylenetetrazole (PTZ)-induced kindling in male rats.Materials and Methods:
In this experimental study, 14 male Wistar rats were allocated into two groups of experimental and control (seven rats in each group). The kindling group received a subconvulsive dose of PTZ (35 mg/kg, intraperitoneally) every 48 hours for 15 sessions, while the control rats were injected with an equal dose of saline. At the end of the injections, rats were decapitated, and their brains were removed. TRPV1 receptor expression in hippocampus region was assessed by real-time polymerase chain reaction method.Results:
The results of the current study showed that expressions of TRPV1 receptors were increased in hippocampus following PTZ-induced kindling in male rats.Conclusions:
Since the expression of TRPV1 receptors increased in hippocampus of epileptic rats, the TRPV1 receptors may be good candidates for epilepsy treatment.Keywords
1. Background
Epilepsy is a prevalent neurological disorder marked by repetitive aberrant electrical activity and by recurrent seizures in the central nervous system. It has considerable impact on the patients’ quality of life and greatly increases the risk of injury, socioeconomic disadvantage, and even mortality (1).
The exact pathogenic mechanisms of epilepsy remain unclear. The association between epilepsy and the hippocampus is well-known and important (2, 3). Patients with temporal lobe epilepsy (TLE), which is the most common form of epilepsy in adults, have focal seizures, some of which with secondary generalization. In most of these patients, the epileptic focus is located in the medial temporal lobe structures, such as the hippocampus, amygdala and parahippocampal gyrus (3). Because of its relatively simple histological construction, it is often used in experimental and clinical studies of this disease.
Transient receptor potential vanilloid type 1 (TRPV1) is a member of a large family of ligand-gated nonselective cation channel that is permeable to many cations, including Ca2+, Na+, and Mg2+ . It is activated by capsaicin, resiniferatoxin, noxious heat (> 43°C) and low pH (4).These channels are expressed in many brain regions with the highest level in the hippocampus and cortex (5). Recently, some researchers showed the role of TRPV1 receptors in seizure and showed that TRPV1 stimulation by capsaicin could act as a proconvulsant chemical (6). Also, we have previously shown that TRPV1 stimulation significantly aggravated the indices of seizure in both models of epileptic seizure (7).
2. Objectives
Based on the above evidence, the present study was designed to determine mRNA expression levels of TRPV1 receptors in the hippocampus following pentylenetetrazole (PTZ)-induced kindling in male rats.
3. Materials and Methods
3.1. Animals and Treatment Groups
Adult male Wistar rats from animal house of Rafsanjan University of Medical Sciences (Iran), weighing 250 - 300 g at the time of study, were used in this study. Animals were housed four per cage, in a room with a 12.12 hours light/dark cycle and controlled temperature (23°C ± 2°C). Animals had access to food and water ad libitum. Animal handling was performed in accordance with EU directive 86/609/EEC for the use of laboratory animals in research and approved by the local ethics committee (the ethics committee of Rafsanjan University of Medical Sciences -ethical code: IR.RUMS.REC.1394.1394.176-). We eliminated all distorting factors to minimize the number of animals used and their suffering.
The rats were randomly allocated into the experimental (kindling) and control groups (seven animals in each group). The kindling group received a subconvulsive dose of PTZ (35 mg/kg, intraperitoneally) every 48 hours for 15 sessions, while the control rats were injected with an equal dose of saline.
3.2. Kindling Model
Pentylenetetrazole was purchased from Sigma-Aldrich (UK) and dissolved in 0.9% sterile saline on a weight/volume basis on the day of use. Subsequent to PTZ injections, animals were monitored for 30 minutes and epileptic behaviors were scored as follows: 0, no behavioral changes; 1, facial movements, ear and whisker twitching; 2, myoclonic convulsions without rearing; 3, myoclonic convulsions with unilateral forelimb clonus; 4, myoclonic convulsions with rearing; 5, loss of posture and generalized clonic-tonic seizures (8).
3.3. Tissue Preparation
At the end of the experiment, mice were sacrificed by decapitation and the brains were immediately removed under the aseptic condition. The hippocampus region was micro-dissected from coronal sections of brain on the ice and immediately stored at -80°C until use.
3.4. RNA Isolation and cDNA Synthesis
Quantitative real-time polymerase chain reaction (RT-PCR) was used to investigate any differences in the expression of TRPV1 gene between the groups. Total RNA was extracted from the frozen samples by Trizol reagent (purchased from Parstous Company, Tehran, Iran) according to the manufacturer’s guidance. The RNA was quantitated spectrophotometrically at 260/280 nm. The 260/280 ratio > 1.8 was considered an acceptable measure of RNA purity.
Five micrograms of RNA were converted to cDNA using a cDNA synthesis kit (Parstous Co., Tehran, Iran) with both oligo (dT) and random hexamer primers. The reverse transcription step and generation of cDNA was performed using the following steps: 70°C for 10 minutes (without reverse transcription enzymes), 20°C for 1 minute (cooling), addition of reverse transcription enzymes, 42°C for 60 minutes, and finally held in 95°C up to 10 minutes to inactivate the reverse transcription enzymes.
3.5. Quantitative Real-Time Polymerase Chain Reaction
Real-time PCR was performed using 10µL of a SYBR green master mix, which was purchased from Parstous Company (Tehran, Iran), combined with 200 ng of template cDNA with 2 µL of appropriate primer (Table 1) in a final volume of 20 µL by a Bio-Rad CFX96 system (Bio-Rad Company, Foster City, USA) using the following program: 1 cycle of 95°C for 15 minute, 40 cycles of 95°C for 30 seconds, and 60°C for 30 seconds and finally 72°C for 30 seconds. Primer were synthesized by the Bionner Company (Korea). Real-time PCR was carried out in triplicate and the β-Actin housekeeping gene was used for normalization of amplification signals of target genes. The relative amounts of PCR product were determined using the 2^ - ΔCt formula. The dissociation stages, melting curves and quantitative analyses of the data were performed using CFX manager software version 1.1.308.111 (Bio-Rad, Foster City, USA).
Gene-Specific Primers for Amplification of Rat Transient Receptor Potential Vanilloid Type 1 and β-Actin mRNAs by Real-Time PCR
| Primer | TRPV1 | β-Actin |
|---|---|---|
| Forward | 5’-ACTCCTGACGGCAAGGATGAC-3’ | 5’-AGAGGGAAATCGTGCGTGAC-3’ |
| Reverse | 5’-ACCCACATTGGTGTTCCAGGTAG-3’ | 5’-CAATAGTGATGACCTGGCCGT-3’ |
3.6. Statistical Analysis
Statistical analysis was performed using Excel and SPSS software version 18. All data are expressed as mean ± SEM. To compare the expression of the TRPV1 mRNA level between the groups, an independent sample t-test was used. Also, repeated measurement ANOVA (RMA) was used to compare the kindling scores in different days of the study. The P value < 0.05 was considered to be statistically significant.
4. Results
4.1. Induction of Seizure
The average of seizure scores in the kindling group increased with the number of PTZ injections (Figure 1). This observation suggests that repeated PTZ injections elicited a progressive increase in seizure severity. Results showed a significant difference in seizure stages among injection sessions (RMA, P < 0.05).
Effects of Pentylenetetrazole - Induced Kindling on TRPV1 Gene Expression

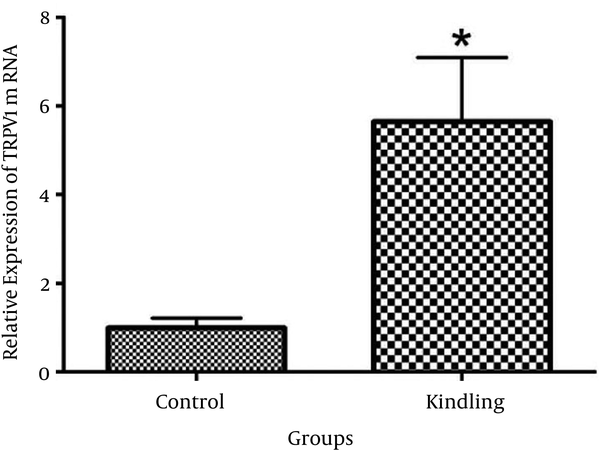
The results showed that mRNA expression levels of TRPV1 significantly increased by 5.64 ± 1.44 fold (P = 0.013) in the hippocampus of rats that received PTZ compared to saline-treated rats (Figure 2).
mRNA Expression Levels of TRPV1 in the Hippocampus of Rats that Received PTZ in Compare With Saline-Treated Rats
5. Discussion
This study was undertaken to evaluate the role of epilepsy on mRNA levels of the TRPV1 receptor in the hippocampus region. The results of the current study showed that following PTZ-induced kindling, TRPV1 receptor mRNA levels increased in the hippocampus compared with saline- treated rats.
TRPV1 receptor is a nonselective cation channel from the TRP family ion channels which are expressed in many regions in the brain including hippocampus (5). It is coupled to ligand-gated ion channels that flux many cations, especially Ca2+ (9). Activation of these receptors could modulate the secretion of excitatory (glutamate) and inhibitory (GABA) neurotransmitters (10) and consequently regulate the excitability of neuronal circuits (11) in some brain areas, especially in hippocampus (10).
There are many reports on the involvement of TRPV1 in the modulation of synaptic plasticity (12), suggesting a role in long-term changes in neural networks. In many neurologic diseases such as epilepsy, Alzheimer’s disease, and Parkinson’s disease (13) synaptic plasticity impairments have been documented. Interestingly, the mechanisms underlying memory modulation by the model of long-term potentiation (LTP) are similar to those underlying epileptogenesis by kindling (14). It is known that TRPV1 activation facilitates LTP and suppresses long-term depression (LTD) in the hippocampus (15). Therefore, it can be hypothesized that the increased expression of TRPV1 may be involved in the pathophysiology of seizure by modulating the synaptic plasticity.
Fu et al. hypothesized that this receptor is a potential target for antiepileptogenesis (12). In line with this hypothesis there are studies that reported the effect of TRPV1 receptors on seizure. Lee et al. showed that capsaicin, a TRPV1 receptor agonist, inhibited PTZ-induced convulsion (16). Chen and colleagues reported that piperine (the principal alkaloid component of pepper) exerts its antiseizure effects via the TRPV1 receptor in mice (17). The role of these receptors in the development of kindling is investigated by our group already. Results demonstrated that activation of TRPV1 receptors by OLDA (N-oleoyldopamine) accelerated the development of PTZ kindling while inhibition of these receptors by AMG-9810 [(E)-3-(4-t-butylphenyl)-N-(2, 3-dihydro-benzo[b][1,4] dioxin-6-yl) acrylamide] retarded the development of PTZ kindling (7).
Also, it was reported that the hyperthermic seizure threshold in TRPV1 mice was significantly higher (41.1°C) compared with wild-type mice (39.5°C). Furthermore, this study showed that heat-triggered neuronal bursting was influenced mainly in the CA1 and CA3 regions of the hippocampus (18). In an interesting study, Sun et al. measured the expression of mRNA and the protein level of TRPV1 in the cortex and hippocampus of patients with mesial TLE. They reported that the expression of TRPV1 was increased in these patients compared with people that have no history of epilepsy (19). Shu et al. reported an over-expression of TRPV1 in cortical lesions from patients with tuberous sclerosis complex and focal cortical dysplasia type IIb, which are recognized as causes of intractable epilepsy (20). Taking these results with the results of this study together, one may suggest that up-regulation of TRPV1 receptors may involve pathogenesis of epilepsy.
TRPV1 receptors seem to have a role in the pathophysiological processes of some other brain-related diseases. In diabetic neuropathic pain that is one of the most common clinical manifestations of diabetes mellitus, the TRPV1 mRNA level was significantly up-regulated in the dorsal root ganglion neurons (21). In lipopolysaccharide-induced fever, the expression of the TRPV1 in the rat preoptic-anterior hypothalamus area was increased (22). In Alzheimer’s disease, it is demonstrated that β-Amyloid induced inflammation in astrocytes lacking fatty acid amide hydrolase via a mechanism involving PPAR-α, PPAR-γ and TRPV1 (23). Some agents that activate cannabinoid system have beneficial effects in rat model of multiple sclerosis through TRPV1 receptors (24).
In conclusion, the findings of the current study show an increased expression of TRPV1 in the hippocampus of PTZ-induced kindling rats. In our previous study we reported that activation of TRPV1 receptors accelerated the development of PTZ kindling while inhibition of these receptors retarded the development of PTZ kindling. Moreover, some studies showed that the TRPV1 receptors increased in human epilepsy. So, the TRPV1 receptors may be good candidates for epilepsy treatment.
Acknowledgements
References
-
1.
Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1(3):173-81.
-
2.
Chatzikonstantinou A. Epilepsy and the hippocampus. Front Neurol Neurosci. 2014;34:121-42.
-
3.
Sendrowski K, Sobaniec W. Hippocampus, hippocampal sclerosis and epilepsy. Pharmacol Rep. 2013;65(3):555-65.
-
4.
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816-24.
-
5.
Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Mol Brain Res. 2005;135(1):162-8.
-
6.
Manna SS, Umathe SN. Involvement of transient receptor potential vanilloid type 1 channels in the pro-convulsant effect of anandamide in pentylenetetrazole-induced seizures. Epilepsy Res. 2012;100(1):113-24.
-
7.
Shirazi M, Izadi M, Amin M, Rezvani ME, Roohbakhsh A, Shamsizadeh A. Involvement of central TRPV1 receptors in pentylenetetrazole and amygdala-induced kindling in male rats. J Neurol Sci. 2014:1-7.
-
8.
Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281-94.
-
9.
Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci STKE. 2001;2001(90).
-
10.
Al-Hayani A, Wease KN, Ross RA, Pertwee RG, Davies SN. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41(8):1000-5.
-
11.
Mori F, Ribolsi M, Kusayanagi H, Monteleone F, Mantovani V, Buttari F. TRPV1 channels regulate cortical excitability in humans. J Neurosci. 2012;32(3):873-9.
-
12.
Fu M, Xie Z, Zuo H. TRPV1: a potential target for antiepileptogenesis. Med Hypotheses. 2009;73(1):100-2. [PubMed ID: 19328632].
-
13.
van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10(3):207-14. [PubMed ID: 20425036]. https://doi.org/10.1007/s11910-010-0104-8.
-
14.
Meador KJ. The basic science of memory as it applies to epilepsy. Epilepsia. 2007;48 Suppl 9:23-5. [PubMed ID: 18047596]. https://doi.org/10.1111/j.1528-1167.2007.01396.x.
-
15.
Li HB, Mao RR, Zhang JC, Yang Y, Cao J, Xu L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol Psychiatry. 2008;64(4):286-92. [PubMed ID: 18405883]. https://doi.org/10.1016/j.biopsych.2008.02.020.
-
16.
Lee TH, Lee JG, Yon JM, Oh KW, Baek IJ, Nahm SS, et al. Capsaicin prevents kainic acid-induced epileptogenesis in mice. Neurochem Int. 2011;58(6):634-40. [PubMed ID: 21333704]. https://doi.org/10.1016/j.neuint.2011.01.027.
-
17.
Chen CY, Li W, Qu KP, Chen CR. Piperine exerts anti-seizure effects via the TRPV1 receptor in mice. Eur J Pharmacol. 2013;714(1-3):288-94. [PubMed ID: 23911889]. https://doi.org/10.1016/j.ejphar.2013.07.041.
-
18.
Kauer JA, Connors BW, Kim JA, Gibson HE. Treatment and prophylaxis of epilepsy and febrile seizures. Google Patents; 2011.
-
19.
Sun FJ, Guo W, Zheng DH, Zhang CQ, Li S, Liu SY, et al. Increased expression of TRPV1 in the cortex and hippocampus from patients with mesial temporal lobe epilepsy. J Mol Neurosci. 2013;49(1):182-93. [PubMed ID: 22936245]. https://doi.org/10.1007/s12031-012-9878-2.
-
20.
Shu HF, Yu SX, Zhang CQ, Liu SY, Wu KF, Zang ZL, et al. Expression of TRPV1 in cortical lesions from patients with tuberous sclerosis complex and focal cortical dysplasia type IIb. Brain Dev. 2013;35(3):252-60. [PubMed ID: 22647236]. https://doi.org/10.1016/j.braindev.2012.04.007.
-
21.
Cui YY, Xu H, Wu HH, Qi J, Shi J, Li YQ. Spatio-temporal expression and functional involvement of transient receptor potential vanilloid 1 in diabetic mechanical allodynia in rats. PLoS One. 2014;9(7):102052. [PubMed ID: 25020137]. https://doi.org/10.1371/journal.pone.0102052.
-
22.
Fan-xin M, Li-mei S, Bei S, Xin Q, Yu Y, Yu C. Heat shock factor 1 regulates the expression of the TRPV1 gene in the rat preoptic-anterior hypothalamus area during lipopolysaccharide-induced fever. Exp Physiol. 2012;97(6):730-40. [PubMed ID: 22427437]. https://doi.org/10.1113/expphysiol.2011.064204.
-
23.
Benito C, Tolon RM, Castillo AI, Ruiz-Valdepenas L, Martinez-Orgado JA, Fernandez-Sanchez FJ, et al. beta-Amyloid exacerbates inflammation in astrocytes lacking fatty acid amide hydrolase through a mechanism involving PPAR-alpha, PPAR-gamma and TRPV1, but not CB(1) or CB(2) receptors. Br J Pharmacol. 2012;166(4):1474-89. [PubMed ID: 22321194]. https://doi.org/10.1111/j.1476-5381.2012.01889.x.
-
24.
Cabranes A, Venderova K, de Lago E, Fezza F, Sanchez A, Mestre L, et al. Decreased endocannabinoid levels in the brain and beneficial effects of agents activating cannabinoid and/or vanilloid receptors in a rat model of multiple sclerosis. Neurobiol Dis. 2005;20(2):207-17. [PubMed ID: 16242629]. https://doi.org/10.1016/j.nbd.2005.03.002.