Abstract
Context:
Radiation-induced lumbosacral plexopathy is still under-recognized although its symptomatology can really lower the quality of life and impair self-sufficient functions of patients often cured of cancer.Objectives:
This review aimed to depict radiation-induced lumbosacral plexopathy through a systematic review of the available literature and discuss various aspects of the clinical management of this pathology.Data Sources:
We searched for all English medical papers registered in Web of Knowledge, PubMed, Google Scholar, and ScienceDirect from January 1990 to November 2018.Study Selection:
From among all articles concerning radiotherapy and lumbosacral plexopathy, we included papers that dealt with human samples and excluded non-human samples and case reports.Results:
Out of 1,312 articles, we selected 42 articles of which 21 met the eligibility criteria and were included in the present analysis. Five papers were general reviews, three focused on the diagnosis of disease, three analyzed the role of different therapies, and the remaining 10 articles concerned the methodology of radiation therapy.Conclusion:
In the next future, we must analyze the dosimetry parameters and the clinical parameters, with appropriate follow-up times, thus providing sufficient data to use for developing organ sparing strategies tailored to individual patients. Therefore, we can reduce this type of side effects that have a huge impact on the quality of life of patients.Keywords
1. Context
In the clinical management of cancer patients, it is of paramount importance to achieve optimal loco-regional control of disease while simultaneously trying to minimize complications (1). Although the plethora of literature has discussed radiation-induced brachial plexopathy and the validated dose constraints (2), radiation-induced lumbosacral plexopathy is still underestimated. This is while its symptomatology can really lower the quality of life and impair self-sufficient function of patients often cured of cancer (3).
2. Objectives
This review aimed to depict radiation-induced lumbosacral plexopathy through a systematic review of the available literature and discuss various aspects of the clinical management of this pathology.
3. Data Sources
The systematic review was conducted by searching for all English medical papers registered in Web of Knowledge, PubMed, Google Scholar, and ScienceDirect from January 1990 to November 2018 (latest search on November 15, 2018).
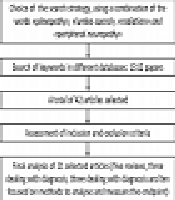
The search strategy included “lumbo-sacral AND radiation” or “plexopathy AND radiation”, or “peripheral AND neuropathy AND radiation” (Figure 1).
Flowchart of the systematic review analysis

We also searched in the reference lists of other reviews, as well as in the references of the selected articles, to retrieve possibly missing papers in the electronic search. We also evaluated the evidence level of each of the selected articles with the PICO format (patient/problem, intervention, comparison, outcome), as illustrated in Table 1.
PICO Format for the Systematic Review
| Definition | Description |
|---|---|
| Patients | Patients developing radiation-induced lumbosacral plexopathy |
| Interventions | Type, pathophysiology, and diagnosis of plexopathy |
| Comparison | The role of different approaches to reduce this side effect, both in the prevention of the event and in the treatment |
| Outcome | Recovery (or reduction of the ratio of the patients) of the lumbosacral plexopathy induced by radiation therapy |
3.1. Inclusion Criteria
Based on the search strategy, we collected all articles concerning radiotherapy and lumbosacral plexopathy. We only included papers that dealt with human samples and excluded non-human samples and case reports (up to three cases for each paper).
Each researcher individually searched the databases, which resulted in the retrieval of 42 articles. However, we excluded 21 articles that met the exclusion criteria. Finally, we used evidence-based medicine resources (EBMR) to assess the scientific level of the evidence.
4. Results
Out of 21 papers included in this review, five were general reviews of lumbosacral plexopathy and radiation-induced neuropathies (3-7), three focused on the diagnosis of disease with imaging and/or electromyography examinations (8-10), three analyzed the role of different therapies (11-13), and the remaining 10 articles concerned the methodology of radiation therapy (dosimetry analysis, contouring guidelines) (14-23) (Table 2).
A Summary of the Descriptions of the Reviewed Papers Dealing with Lumbosacral Plexopathy
| First Author | Year | Type | Description |
|---|---|---|---|
| Dyck (4) | 2014 | Review | Description of clinical features, pathogenesis, and management of different types of lumbosacral plexopathy |
| Pradat (5) | 2013 | Review | Physiopathology of radiation-induced peripheral nerve damage |
| Delanian (3) | 2012 | Review | Review of radiation-induced neuropathy in cancer survivors |
| Forman (6) | 1990 | Review | Description of peripheral neuropathy in cancer: clinical types, etiology, and presentation |
| Dropcho (7) | 2010 | Review | Clinical features, diagnosis, and management options for patients with radiation neurotoxicity |
| Rash (14) | 2015 | Original Article | Analysis of dose delivered to the lumbosacral plexus although the dose threshold for radiation-induced neuropathy remained undefined. |
| Tunio (15) | 2014 | Original Article | Evaluation of dose distribution and correlation with radiation-induced lumbosacral plexopathy |
| Yi (17) | 2012 | Original Article | Development of a standardized method for contouring the lumbosacral plexus |
| Min (16) | 2014 | Original Article | External validation of the method developed by Yi, 2012 |
| Frykholm (18) | 1996 | Original Article | Retrospective analysis of the individual treatment of patients with rectal adenocarcinoma developing acute pain and subacute neurological symptoms |
| Georgiou (19) | 1993 | Original Article | Clinical findings and dosimetric analysis of radiation-induced lumbosacral plexopathy in gynecologic tumors |
| Stubblefield (20) | 2017 | Original Article | Determination of the percentage of high-dose single-fraction stereotactic radiosurgery to the spine that resulted in peripheral nerve injury. |
| Brydoy (21) | 2007 | Original Article | Estimation of the rate of neurological adverse effects following radiotherapy for testicular seminoma and analysis of possible dose-related effects. |
| Ratto (22) | 1997 | Original Article | Analysis of late effects of different therapies on lumbosacral plexus and anorectal physiology in patients treated for rectal cancer |
| Lim (23) | 2006 | Original Article | Analysis of preoperative chemoradiation for rectal cancer and correlation with pudendal neuropathy |
| Ko (8) | 2011 | Diagnosis | Clinical and electrophysiological findings in adult patients with non-traumatic plexopathies |
| van Alfen (9) | 2013 | Diagnosis | Diagnosis of brachial and lumbosacral plexus lesions, with a focus on clinical examination |
| Jaeckle (10) | 2010 | Diagnosis | Differential diagnosis between neoplastic and radiation-induced plexopathy, based on clinical, neuroimaging, and electrophysiological features |
| Glantz (11) | 1994 | Therapies | Experimental treatment with heparin and warfarin for radiation-induced nervous system damage |
| Schiano di Visconte (12) | 2018 | Therapies | Sacral nerve stimulation in fecal incontinence after multimodal therapies for pelvic malignancies |
| Delanian (13) | 2008 | Therapies | Experimental therapy of radiation-induced lumbosacral plexopathy with pentoxifylline, tocopherol, and clodronate combination |
In the discussion section, we will briefly comment on various aspects of radiation-induced lumbosacral plexopathy, starting from anatomy of lumbosacral plexus, followed by the pathophysiology of the damage, diagnosis, current strategies to preserve lumbosacral plexus, and future challenges.
5. Discussion
5.1. Anatomy
The lumbosacral plexus is anatomically divided into upper lumbar plexus and lower lumbosacral plexus (24-26). The lumbar plexus originates from the L1 - L4 nerve roots, and is located in the retroperitoneum behind the psoas muscle. The primary anterior branches divide into anterior and posterior branches and the plexus terminates into six major branches: iliohypogastric, ilioinguinal, genitofemoral, femoral, and lateral femoral cutaneous nerves (posterior division), and obturator nerves (anterior division). The first three nerves supply motor and sensory innervation to the abdominal wall, superior gluteal region, groin, and external genitalia while the next three ones innervate the anterior and medial sides of the thigh. The femoral nerve, similarly, terminates into the saphenous nerve that provides sensation to the medial aspect of the leg. The seventh branch is a contribution from L4 to the sacral plexus (24-26). The latter, composed of primary ventral branches from the L5 - S3 levels (divided into anterior and posterior divisions), is located in the back of the small pelvis between the piriformis muscle and the pelvic fascia. The five main terminal nerves are superior and inferior gluteal, posterior femoral cutaneous, pudendal, and sciatic nerves. The sciatic nerve (common peroneal and tibial branches in the thigh), gives innervation to the knee flexors (hamstrings: semimembranosus, semitendinosus, and long and short heads of the biceps femoris), the lateral division of the adductor Magnus muscle, and all muscles innervated by the peroneal and tibial nerves (24-26). It also provides sensory innervation into the entire lower leg below the knee, except for the medial calf, which is innervated by the saphenous nerve. The superior and inferior gluteal nerves supply the innervations of gluteus minimus, medius, and maximus and tensor fascia lata muscles, while the posterior femoral cutaneous nerve gives the sensory innervation to posterior thigh, scrotum/labia, proximal calf, and lower border of gluteus maximus (24-26).
5.2. Pathophysiology
Historically, as described in the related literature, peripheral nerves were considered relatively radioresistant, mainly due to their low mitotic index and low metabolism (27), though the main reason was the short follow-up of radiation therapy patients; thus, this side effect was not commonly reported. Nowadays, as many patients undergoing radiation therapy for pelvic malignancies have good prognosis and the quality of life outcomes are of paramount importance in oncology, this particular type of side effect needs to be taken into serious consideration.
The early effects of radiation exposure start some hours after the event, and include enzyme alterations, bioelectrical changes, abnormal microtubules, and altered permeability of the vascular structures (27, 28). Mendes et al. illustrated two phases of this process. The first phase included electrophysiology and histochemistry alterations and the second phase was strictly correlated with the radiation-induced fibrosis of the surrounding connective tissue (28). Radiation-induced lumbosacral plexopathy has been reportedin the treatment of different pelvic malignancies, especially small pelvic tumors, including the tumors of reproductive organs, testis, rectum, lymphomas, and tumors involving para-aortic nodes (3).
The anatomopathological changes in radiation-induced plexopathy include necrosis and hyalinization of the media of small arteries, as well as the fibrosis of nerves, demyelination, and thickening of epineurium and perineurium (27, 29). The inflammatory response has a pivotal role, like fibroblasts and various infiltrating inflammatory cells, as well as extracellular matrix components, are found in the surrounding connective tissue (30). The physiologyof radiation-induced damage is yet not clear. It seems to rely on the total dose, dose per fraction, irradiation technique, and the use of brachytherapy (15). Since this type of side effects is observed both at doses lower than 60 Gy and at higher doses (31), it seems there are also effective factors not related to radiation therapy (RT). Specifically, these factors could be divided in treatment-related factors, including the surgery especially when it includes extended lymphadenectomy and the use of neurotoxic chemotherapy (cisplatin, taxanes, vinca alkaloids) and patient-related factors, including younger age or advanced age, obesity, diabetes, arthritis, smoking, alcohol consumption, pre-existing collagen vascular diseases, and hypersensibility of patients to radiation damage (3).
5.3. Diagnosis
The diagnosis can be very challenging in the clinical management of patients with suspicious radiation-induced lumbosacral plexopathy. It is important, first, to exclude a recurrence of cancer disease, lumbosacral plexopathy due to different etiologies (diabetic amyotrophic, inflammatory plexitis, retroperitoneal hemorrhage), others neurological disorders (lumbosacral radiculopathy, motoneuron disease, mononeuropathies), and other radiation-induced side effects (mainly on the pelvic bones) (8-10, 32, 33). Computed tomography (CT) and/or magnetic resonance imaging (MRI) are mainly used for this purpose, as they can show metastases infiltrating the plexus, lymphadenopathy, bone erosions, and so on (34). Although a distinctive and pathognomonic imaging appearance of plexopathy is still not clear, MRI can show patchy or multinodular enhancement along the cauda equina and the conus medullaris, appearing to correlate with this type of damage (35, 36).
5.4. Clinical and Electrodiagnostic Findings
Clinical examination and electrodiagnostic tests can be helpful in the diagnostic assessment of lumbosacral plexopathies, especially in cases of difficult differential diagnosis. The onset of neurological signs and symptoms is usually insidious mostly with a sensory-motor damage. Acute and transient symptoms may also appear during or soon after irradiation (21). Patients mainly refer to walking difficulties, with asymmetric bilateral leg painless weakness (80%) (37), starting from 1 - 3 months to years after the completion of radiotherapy, accompanied by amyotrophy and fasciculations. Pain can be present in the lumbar region or in the lower limbs, but usually not early or severe; moreover, there are sensory disturbances such as paresthesias, numbness, and dysesthesias, according to the dermatome involved. In lumbar plexus damage, the knee reflex is reduced, with sensory loss in the L2 - L4 dermatomes, weakness of hip flexors, knee extensors, and leg adductors. A lesion of the sacral plexus often causes symptoms similar to sciatic nerve lesions, with further involvement of gluteal muscles and sometimes, of the anal sphincter, with sensory symptoms in the pelvis, posterior aspect of thigh, lateral calf, and foot. Sphincter disorders, when present, can be due to peripheral neurogenic damage or pelvic fibrosis. These disturbances are usually slowly progressive; some of them are irreversible, dramatically impairing the quality of life and causing mood disorders. Some authors described stabilization or progression of neurological symptoms over a few months or a few years while others reported rare cases of neurological improvement (10, 38-40). In this scenario, electrophysiological studies are crucial to localize the site of injury and discriminate the etiology.
The sensory nerve action potential (SNAP) amplitude may be decreased in lesions affecting the plexus while in cases of lesions proximal to the dorsal root ganglia., such as radiculopathies and nerve root avulsions, SNAP parameters are normal, even in the presence of sensory loss. A decreased compound muscle action potential (CMAP) amplitude on the affected side compared to the normal side is generally a better indicator of extensive axonal loss and severe injury.
Needle electromyography (EMG) abnormalities generally include abnormalities in denervation activity at rest (fibrillation potentials, positive sharp waves) and neurogenic recruitment at full effort (reduced motor unit action potentials with increased firing frequency), often accompanied by fasciculations, in the myotomes supplied by the anterior rami of multiple spinal nerves, with sparing of paraspinal muscles. A highly characteristic radiation-induced damage finding is the presence of myokymic discharges, which are absent in direct tumornot present in direct tumor invasion of the plexus (8, 37, 41).
The evaluation of paraspinal muscle is mandatory to rule out lumbosacral radiculopathy because abnormalities in these muscles place the lesion on root levels. In mononeuropathy, abnormalities are limited to one nerve, whereas in plexopathy, more than one nerve is involved. In cases of pure motor-onset lumbosacral plexopathies, the most common differential diagnosis is amyotrophic lateral sclerosis, which is then excluded for the lack of rapid progression of the motor involvement in new territories, the absence of sensory disturbances/electrodiagnostic abnormalities, and pyramidal signs (3). Finally, it is important to underline that electrodiagnostic study results could be normal in acute plexopathy, especially during the first week; therefore, it is recommended waiting at least three weeks before assessing for these typical findings.
5.5. Current Strategies and Future Challenges
At present, the treatment of radiation-induced lumbosacral plexopathy is only symptomatic, as a curative strategy is still not defined. The pain, if present, is treated with non-opioid analgesics, benzodiazepines, tricyclic antidepressants, and antiepileptics, whereas carbamazepine could be used to reduce nerve hyperexcitability, like myokymia (3); however, the role of surgery is still not established.
Vitamins B1 - B6 are often used in clinical practice. Physical therapy is advised for the maintenance of muscular function. It is also very important to avoid the carrying of heavy loads and to avoid movements in extension that could stretch the suffering plexus (3). Experimental treatments are currently under investigation, such as heparin and warfarin, combined pentoxifylline and tocopherol alone or in combination with clodronate (PENTOCLO) (11, 13).
As always in medicine, the best approach could be prevention by reducing the total dose, the dose per fraction, and the RT volumes, especially in high-risk patients. In this regard, it is noteworthy to underline that currently in clinical trials, the lumbosacral plexus is still not defined as an organ at risk (OAR). Thus, in spite of the use of more advanced techniques of radiation therapy, such as intensity modulated radiotherapy (IMRT), this organ could undergo unwanted “dose dumping”. Yi et al. tried to standardize a method for contouring the lumbosacral plexus, but unfortunately, it is currently not adopted at most RT Departments, as well as in clinical trials (16, 17).
5.6. Conclusions
Radiation-induced lumbosacral plexopathy is actually under-recognized although its symptomatology can really compromise the quality of life of patients often cured of cancer. In the next future, it is of paramount importance to start studying this type of RT-induced damage, including the dosimetry parameters (total dose, dose per fraction, and dose-volume histograms of the whole organ according the standardized contouring) and clinical parameters (sex, age, cancer, smoking status, and comorbidities) with an appropriate follow-up time, thus providing sufficient data to develop organ sparing strategies tailored to individual patients.
References
-
1.
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10-9. [PubMed ID: 20171502]. [PubMed Central ID: PMC4041542]. https://doi.org/10.1016/j.ijrobp.2009.07.1754.
-
2.
Bajrovic A, Rades D, Fehlauer F, Tribius S, Hoeller U, Rudat V, et al. Is there a life-long risk of brachial plexopathy after radiotherapy of supraclavicular lymph nodes in breast cancer patients? Radiother Oncol. 2004;71(3):297-301. [PubMed ID: 15172145]. https://doi.org/10.1016/j.radonc.2004.03.005.
-
3.
Delanian S, Lefaix JL, Pradat PF. Radiation-induced neuropathy in cancer survivors. Radiother Oncol. 2012;105(3):273-82. [PubMed ID: 23245644]. https://doi.org/10.1016/j.radonc.2012.10.012.
-
4.
Dyck PJ, Thaisetthawatkul P. Lumbosacral plexopathy. Continuum (Minneap Minn). 2014;20(5 Peripheral Nervous System Disorders):1343-58. [PubMed ID: 25299286]. https://doi.org/10.1212/01.CON.0000455877.60932.d3.
-
5.
Pradat PF, Delanian S. Late radiation injury to peripheral nerves. Handb Clin Neurol. 2013;115:743-58. [PubMed ID: 23931813]. https://doi.org/10.1016/B978-0-444-52902-2.00043-6.
-
6.
Forman A. Peripheral neuropathy in cancer patients: Clinical types, etiology, and presentation. Part 2. Oncology (Williston Park). 1990;4(2):85-9. [PubMed ID: 2167114].
-
7.
Dropcho EJ. Neurotoxicity of radiation therapy. Neurol Clin. 2010;28(1):217-34. [PubMed ID: 19932383]. https://doi.org/10.1016/j.ncl.2009.09.008.
-
8.
Ko K, Sung DH, Kang MJ, Ko MJ, Do JG, Sunwoo H, et al. Clinical, electrophysiological findings in adult patients with non-traumatic plexopathies. Ann Rehabil Med. 2011;35(6):807-15. [PubMed ID: 22506209]. [PubMed Central ID: PMC3309383]. https://doi.org/10.5535/arm.2011.35.6.807.
-
9.
van Alfen N, Malessy MJ. Diagnosis of brachial and lumbosacral plexus lesions. Handb Clin Neurol. 2013;115:293-310. [PubMed ID: 23931788]. https://doi.org/10.1016/B978-0-444-52902-2.00018-7.
-
10.
Jaeckle KA. Neurologic manifestations of neoplastic and radiation-induced plexopathies. Semin Neurol. 2010;30(3):254-62. [PubMed ID: 20577932]. https://doi.org/10.1055/s-0030-1255219.
-
11.
Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SJ. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44(11):2020-7. [PubMed ID: 7969953]. https://doi.org/10.1212/wnl.44.11.2020.
-
12.
Schiano di Visconte M, Santoro GA, Cracco N, Sarzo G, Bellio G, Brunner M, et al. Effectiveness of sacral nerve stimulation in fecal incontinence after multimodal oncologic treatment for pelvic malignancies: A multicenter study with 2-year follow-up. Tech Coloproctol. 2018;22(2):97-105. [PubMed ID: 29313165]. https://doi.org/10.1007/s10151-017-1745-2.
-
13.
Delanian S, Lefaix JL, Maisonobe T, Salachas F, Pradat PF. Significant clinical improvement in radiation-induced lumbosacral polyradiculopathy by a treatment combining pentoxifylline, tocopherol, and clodronate (Pentoclo). J Neurol Sci. 2008;275(1-2):164-6. [PubMed ID: 18804790]. https://doi.org/10.1016/j.jns.2008.08.004.
-
14.
Rash D, Durbin-Johnson B, Lim J, Dieterich S, Huddleston A, Yi S, et al. Dose delivered to the lumbosacral plexus from high-dose-rate brachytherapy for cervical cancer. Int J Gynecol Cancer. 2015;25(5):897-902. [PubMed ID: 25768077]. [PubMed Central ID: PMC4563992]. https://doi.org/10.1097/IGC.0000000000000427.
-
15.
Tunio M, Al Asiri M, Bayoumi Y, Abdullah OA, AlHameed M, Gabriela SL, et al. Lumbosacral plexus delineation, dose distribution, and its correlation with radiation-induced lumbosacral plexopathy in cervical cancer patients. Onco Targets Ther. 2015;8:21-7. [PubMed ID: 25565862]. [PubMed Central ID: PMC4278780]. https://doi.org/10.2147/OTT.S71086.
-
16.
Min M, Roos D, Keating E, Kerr L, Mukherjee R, Potter A, et al. External validation of the lumbosacral plexus-contouring protocol developed by Yi et al. (IJROBP 2012; 84: 376-82) for pelvic malignancies. J Med Imaging Radiat Oncol. 2014;58(1):117-24. [PubMed ID: 24529065]. https://doi.org/10.1111/1754-9485.12106.
-
17.
Yi SK, Mak W, Yang CC, Liu T, Cui J, Chen AM, et al. Development of a standardized method for contouring the lumbosacral plexus: a preliminary dosimetric analysis of this organ at risk among 15 patients treated with intensity-modulated radiotherapy for lower gastrointestinal cancers and the incidence of radiation-induced lumbosacral plexopathy. Int J Radiat Oncol Biol Phys. 2012;84(2):376-82. [PubMed ID: 22342301]. https://doi.org/10.1016/j.ijrobp.2011.11.074.
-
18.
Frykholm GJ, Sintorn K, Montelius A, Jung B, Pahlman L, Glimelius B. Acute lumbosacral plexopathy during and after preoperative radiotherapy of rectal adenocarcinoma. Radiother Oncol. 1996;38(2):121-30. [PubMed ID: 8966224]. https://doi.org/10.1016/0167-8140(95)01665-1.
-
19.
Georgiou A, Grigsby PW, Perez CA. Radiation induced lumbosacral plexopathy in gynecologic tumors: Clinical findings and dosimetric analysis. Int J Radiat Oncol Biol Phys. 1993;26(3):479-82. [PubMed ID: 8514543]. https://doi.org/10.1016/0360-3016(93)90966-y.
-
20.
Stubblefield MD, Ibanez K, Riedel ER, Barzilai O, Laufer I, Lis E, et al. Peripheral nervous system injury after high-dose single-fraction image-guided stereotactic radiosurgery for spine tumors. Neurosurg Focus. 2017;42(3). E12. [PubMed ID: 28245730]. [PubMed Central ID: PMC5546836]. https://doi.org/10.3171/2016.11.FOCUS16348.
-
21.
Brydoy M, Storstein A, Dahl O. Transient neurological adverse effects following low dose radiation therapy for early stage testicular seminoma. Radiother Oncol. 2007;82(2):137-44. [PubMed ID: 17189656]. https://doi.org/10.1016/j.radonc.2006.11.022.
-
22.
Ratto C, Sofo L, Valentini V, Genovesi D, Morganti AG, Verna L, et al. Late effects of integrated therapies, including IORT, on lumbosacral plexus and anorectal physiology in patients treated for rectal cancer. Front Radiat Ther Oncol. 1997;31:41-4. [PubMed ID: 9263785]. https://doi.org/10.1159/000061141.
-
23.
Lim JF, Tjandra JJ, Hiscock R, Chao MW, Gibbs P. Preoperative chemoradiation for rectal cancer causes prolonged pudendal nerve terminal motor latency. Dis Colon Rectum. 2006;49(1):12-9. [PubMed ID: 16292664]. https://doi.org/10.1007/s10350-005-0221-7.
-
24.
Hollinshead WH. Anatomy for surgeons. 3. William Henry Hollinshead; 1982.
-
25.
Gebarski KS, Gebarski SS, Glazer GM, Samuels BI, Francis IR. The lumbosacral plexus: Anatomic-radiologic-pathologic correlation using CT. Radiographics. 1986;6(3):401-25. [PubMed ID: 2825251]. https://doi.org/10.1148/radiographics.6.3.2825251.
-
26.
Mirilas P, Skandalakis JE. Surgical anatomy of the retroperitoneal spaces, Part IV: Retroperitoneal nerves. Am Surg. 2010;76(3):253-62. [PubMed ID: 20349652].
-
27.
Gillette EL, Mahler PA, Powers BE, Gillette SM, Vujaskovic Z. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys. 1995;31(5):1309-18. [PubMed ID: 7713790]. https://doi.org/10.1016/0360-3016(94)00422-H.
-
28.
Mendes DG, Nawalkar RR, Eldar S. Post-irradiation femoral neuropathy. A case report. J Bone Joint Surg Am. 1991;73(1):137-40. [PubMed ID: 1985985]. https://doi.org/10.2106/00004623-199173010-00020.
-
29.
Moore NR, Dixon AK, Wheeler TK, Freer CE, Hall LD, Sims C. Axillary fibrosis or recurrent tumour. An MRI study in breast cancer. Clin Radiol. 1990;42(1):42-6. [PubMed ID: 2390836]. https://doi.org/10.1016/s0009-9260(05)81621-6.
-
30.
Hoeller U, Bonacker M, Bajrovic A, Alberti W, Adam G. Radiation-induced plexopathy and fibrosis. Is magnetic resonance imaging the adequate diagnostic tool? Strahlenther Onkol. 2004;180(10):650-4. [PubMed ID: 15480514]. https://doi.org/10.1007/s00066-004-1240-3.
-
31.
Cai Z, Li Y, Hu Z, Fu R, Rong X, Wu R, et al. Radiation-induced brachial plexopathy in patients with nasopharyngeal carcinoma: A retrospective study. Oncotarget. 2016;7(14):18887-95. [PubMed ID: 26934119]. [PubMed Central ID: PMC4951337]. https://doi.org/10.18632/oncotarget.7748.
-
32.
Nardone V, Tini P, Carbone SF, Grassi A, Biondi M, Sebaste L, et al. Bone texture analysis using CT-simulation scans to individuate risk parameters for radiation-induced insufficiency fractures. Osteoporos Int. 2017;28(6):1915-23. [PubMed ID: 28243706]. https://doi.org/10.1007/s00198-017-3968-5.
-
33.
Nardone V, Tini P, Croci S, Carbone SF, Sebaste L, Carfagno T, et al. 3D bone texture analysis as a potential predictor of radiation-induced insufficiency fractures. Quant Imaging Med Surg. 2018;8(1):14-24. [PubMed ID: 29541619]. [PubMed Central ID: PMC5835655]. https://doi.org/10.21037/qims.2018.02.01.
-
34.
Taylor BV, Kimmel DW, Krecke KN, Cascino TL. Magnetic resonance imaging in cancer-related lumbosacral plexopathy. Mayo Clin Proc. 1997;72(9):823-9. [PubMed ID: 9294528]. https://doi.org/10.4065/72.9.823.
-
35.
Hsia AW, Katz JS, Hancock SL, Peterson K. Post-irradiation polyradiculopathy mimics leptomeningeal tumor on MRI. Neurology. 2003;60(10):1694-6. [PubMed ID: 12771271]. https://doi.org/10.1212/01.wnl.0000063320.61458.d8.
-
36.
Ducray F, Guillevin R, Psimaras D, Sanson M, Mokhtari K, Delanian S, et al. Postradiation lumbosacral radiculopathy with spinal root cavernomas mimicking carcinomatous meningitis. Neuro Oncol. 2008;10(6):1035-9. [PubMed ID: 18755918]. [PubMed Central ID: PMC2719001]. https://doi.org/10.1215/15228517-2008-069.
-
37.
Thomas JE, Cascino TL, Earle JD. Differential diagnosis between radiation and tumor plexopathy of the pelvis. Neurology. 1985;35(1):1-7. [PubMed ID: 2981416]. https://doi.org/10.1212/wnl.35.1.1.
-
38.
Coulombe G, Thiessen B, Balkwill S, Aquino-Parsons C. Polyradiculopathy post-concomitant chemoradiation for carcinoma of the uterine cervix treated with pelvic and para-aortic fields. Gynecol Oncol. 2005;99(3):774-7. [PubMed ID: 16188304]. https://doi.org/10.1016/j.ygyno.2005.08.005.
-
39.
Pettigrew LC, Glass JP, Maor M, Zornoza J. Diagnosis and treatment of lumbosacral plexopathies in patients with cancer. Arch Neurol. 1984;41(12):1282-5. [PubMed ID: 6093750]. https://doi.org/10.1001/archneur.1984.04050230068022.
-
40.
Jaeckle KA, Young DF, Foley KM. The natural history of lumbosacral plexopathy in cancer. Neurology. 1985;35(1):8-15. [PubMed ID: 2981417]. https://doi.org/10.1212/wnl.35.1.8.
-
41.
Pieters RS, Niemierko A, Fullerton BC, Munzenrider JE. Cauda equina tolerance to high-dose fractionated irradiation. Int J Radiat Oncol Biol Phys. 2006;64(1):251-7. [PubMed ID: 15993548]. https://doi.org/10.1016/j.ijrobp.2005.04.019.