Abstract
Background:
Rotavirus (RV) is associated with diarrhea in children under 5 years old. It leads to severe dehydration. RV infection is the third cause of hospitalization and death in children under 5 years old.Objectives:
This study aimed to assess the frequency of RV infection in hospitalized children under 5 years old with diarrhea during 2021-2022.Methods:
In this cross-sectional observational study, a total of 190 stool samples from hospitalized children with diarrhea were collected in Mofid Children’s Hospital in Tehran from December 2020 to March 2021. RV infection was detected by an enzyme-linked immunosorbent assay (ELISA). Chi-square tests were performed to determine the difference in age and gender group, time, and symptoms.Results:
The overall prevalence of RV infection was 28.5% and higher in boys (68.5%), children aged ≤ 12 months (44.4%), and children with mixed feeding (33.3%); it is more common in winter. Vomiting (79.6%), fever (87.03%), and non-exudative stool (88.8%) were observed in most children with RV, but there were no significant differences in children with and without RV.Conclusions:
Due to the prevalence of RV among children under 5 years of age, establishing a national RV registration system and control programs, like vaccination, seems to be considered.Keywords
1. Background
Rotavirus (RV) is a highly contagious and easily transmitted infection and the leading cause of diarrhea in children under 5 years of age worldwide (1). According to the World Health Organization (WHO), there are more than 25 million outpatient visits and more than 2 million hospitalizations for RV infections each year (2). According to a study, 66.7% of diarrhea-related deaths in children younger than 5 years are due to RV infection (1).
RV enters the mouth and then the intestine through contaminated hands. Infection of the small intestinal epithelial cells leads to severe diarrhea (3). It often leads to severe dehydration, which is responsible for one-third of hospitalization and death due to the infection (1). RV and its complications and mortality can be a public health issue (1), especially in developing countries where before the age of 12 months, three-quarters of children experience their first course of RV diarrhea. Severe RV gastroenteritis is more common in children 6 to 24 months of age, according to the WHO report (2). Studies showed a similar RV prevalence, about 30% to 50% worldwide, among hospitalized children with diarrhea. However, it seems that more than 90% of children with fatal RV infections live in low-income countries (4). According to the literature, RVs are responsible for 20% of deaths from diarrhea in children under 5 years old in developing countries (5). In Iran, the prevalence of RV was estimated at 39.9% between 1986 and 2011 (5).
Chemically, RV multiplies in the cells of the intestinal villi. This proliferation reduces the intestine’s ability to absorb salt and water. Clinically, fever, nausea, and diarrhea are the first symptoms of RV infection. Then, abdominal muscle cramps and recurrent watery diarrhea appear, which may last 3 to 8 days. Also, dehydration is the most widely recognized cause of death from RV, as it is more typical than bacterial infections (3, 6). Reinfection is common in RV infections, but the severity of subsequent infection decreases with each infection because of the immune response. RV is a seasonal infection and is more common in winter (6, 7).
There is no specific treatment for RV except fluid and electrolyte replacement to prevent complications. Also, RV vaccination is recommended to prevent morbidity and mortality (1, 6, 8).
Despite the production and introduction of the RV vaccine, there are some problems regarding its use and effectiveness, such as the cost of its preparation, genetic diversity of different populations, health programs, and pathogenicity of the virus. Obtaining information about the epidemiological characteristics, disease burden, and frequency of RV infection is the first prerequisite for finding appropriate solutions to these problems.
2. Objectives
This study aimed to investigate the frequency of RV infection in hospitalized children ≤ 5 years with diarrhea during 2021 - 2022.
3. Methods
This cross-sectional observational study was performed on children under 5 years old hospitalized for acute diarrhea (liquid or semi-liquid evacuations 3 or more times within 24 hours, and/or 2 times vomiting within 24 hours lasted for up to 14 days) in Mofid Children’s Hospital in Tehran Province, Iran, from December 2020 to March 2022. Due to the fact that the most common symptoms of RV are fever, nausea, and diarrhea, these 3 symptoms were investigated in this study. A pediatric infectious disease specialist or pediatrician was the focal point of the project and oversaw the enrollment of children in the study.
Children older than 5 years, children with diarrhea whose fecal samples were collected more than 48 hours after admission (due to nosocomial infection), and children with incomplete forms and prolonged diarrhea (more than 14 days) were excluded. Despite the importance of fecal consistency in this study, it should be noted that children with exclusive breastfeeding have watery stools.
Demographic and nutritional information, symptoms, the number of hospitalization days for diarrhea treatment, and intensive care unit (ICU) hospitalization reports were recorded at admission and discharge in a questionnaire. Mixed feeding was recorded when supplementing breastfeeding with infant formula had done, while varied nutrition was considered when children ate a wide variety of different foods from the different food groups. All children in the study were divided into the exudative (the existence of blood or ≥ 3 leukocytes per high-power field in 4 or more fields) and non-exudative (lack of blood and leukocytes in the high-power field on a stained microscopic slide) groups in terms of stool samples. At the first contact with the patient, 5 to 10 mL of the stool sample was collected and instantly sent to the laboratory of the hospital research center for examination by a Rotavirus antigen kit (ProSpecT Rotavirus Test Oxoid, UK). The specimens were stored in a refrigerator at 2 - 8°C for up to 7 days until the RV test.
This study is part of the results of the ROTAVICH project that was done by the Pediatric Infections Research Center, Research Institute for Children’s Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran (registration number: 2021/1133866-0) according to the WHO program for “Enhancing Rotavirus Surveillance System & Analytic Reporting.” The study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (code: IR.SBMU.RICH.REC.1400.020).
All data were analyzed using SPSS 26 (SPSS Inc., Chicago, Ill, USA). Numbers and percentages were used to describe nutritional status variables, enzyme-linked immunosorbent assay (ELISA) results, age groups, gender, stool type, and seasons. The chi-square test was used to determine the correlation between variables. P values less than 0.05 were considered statistically significant.
4. Results
In total, 300 children under 5 years old with diarrhea referred to Mofid Children’s Hospital from December 2020 to March 2022 enrolled in this study. Unapproved samples, due to low quality, inappropriate samples, and those with incomplete medical records (110 samples), were excluded from the study. Finally, 190 samples of stool samples were tested by ELISA for RV. The age and gender distribution of the study samples are presented in Table 1. In addition, 57.4% (n = 109) of children were boys, and 42.6% (n = 81) were girls.
The Results of the Rotavirus Enzyme-Linked Immunosorbent Assay in Hospitalized Children by the Age Group a
| RV-ELISA Result | Total | Age Group (mo) | P Value | Gender Group | P Value | Stool Type | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 12 | 13 - 24 | 25 - 36 | 37 - 48 | 49 - 60 | Girl | Boy | Exudative b | Non-exudative c | P Value d | ||||
| Negative | 134 (70.5) | 45 (33.6) | 40 (29.9) | 27 (20.1) | 9 (6.7) | 13 (9.7) | 64 (47.8) | 70 (52.2) | 34 (25.6) | 99 (74.4) | 0.032 | ||
| Positive | 54 (28.5) | 24 (44.4) | 15 (27.8) | 7 (13.0) | 5 (9.3) | 3 (5.6) | 0.036 | 17 (31.5) | 37 (68.5) | 0.04 | 6 (11.3) | 47 (88.7) | |
| Unknown | 2 (1.0) | 0 (0) | 2 (100) | ||||||||||
| Total | 190 | 69 (36.3) | 57 (30.0) | 34 (17.9) | 14 (7.4) | 16 (8.4) | 81 (42.6) | 109 (57.4) | 40 (21.3) | 148 (78.7) | |||
According to Table 1, 36% (n = 69) of children aged ≤ 12 months (40% boys and 31% girls), followed by 13 - 24 months (30%, n = 57), 25 - 36 months (18%, n = 34), 37 - 48 months 7% (n = 14), and 49 - 60 months (9%, n = 16). Thus, the longest hospital stay belonged to ≤ 12 months, and the shortest one’s belonged to the 37 - 48 months age group.
The median of hospitalization days was 3 days (34%), and the average hospital stay for the infected children was 2 - 3 days. The average number of hospitalization days was between 3 - 4 days, most of which were for 2 and 3 days, and there was no difference between the 2 groups.
4.1. RV Status, Stool Types, and Age/Gender Groups
Of the 190 children with acute diarrhea, 28.5% (n = 54) were positive for RV-ELISA; 68.5% of boys and 31.5% of girls had RV-ELISA positive (RV+) results. The ELISA result was unknown in 1% of children (n = 2). In terms of the age group, the highest prevalence of RV+ was observed in ≤12 months (44.4%, n = 24), followed by 13-24 months (27.8%, n = 15). The lowest prevalence of RV+ was observed in 49-60 months (5.6%, n = 3).
Based on the findings, the stool form was semi-liquid in 91.7% of cases and watery in 7.3%. Table 1 shows a comparison of the stool type between the 2 groups. By stool types, 88.7% of the RV+ group had non-exudative stools, while 74.4% of the RV– group had non-exudative stools. Also, exudative stools were observed in 11.3% of the RV+ group and 25.6% of the RV– group. No significant difference was found between the 2 groups regarding the stool type (P ≤ 0.07).
Details of data about the ELISA results for RV infection based on age, gender, and stool forms in symptomatic patients are presented in Table 1.
4.2. RV and Nutritional Status
The nutritional status of children admitted with acute diarrhea in different age groups referred to Mofid Children’s Hospital is shown in Table 2. As can be seen, children over 36 months are placed in the same group as 49 - 60 months because they eat family meals. Of the 190 hospitalized children, 23.3% (n = 44) had exclusive feeding, and 40.6% (n = 28) were under 1 year old. According to the findings, 33.7% (n = 64) of children used mixed nutrition.
The Nutritional Status of Children Admitted with Acute Diarrhea in Different Age Groups
| Variable | Total (N) | Nutritional Status | P Value | |||
|---|---|---|---|---|---|---|
| Exclusively Breastfeed | Formula | Mixed b | Varied Nutrition b | |||
| ELISA | 0.72 | |||||
| Negative | 134 | 34 (25.4) | 32 (23.9) | 45 (33.6) | 23 (17.2) | |
| Positive | 54 | 10 (18.5) | 16 (29.6) | 18 (33.3) | 10 (18.5) | |
| Age (mon) | < 0.0001 | |||||
| ≤ 12 | 69 | 28 (40.6) | 24 (34.8) | 17 (24.6) | 0 (0) | |
| 13 - 24 | 57 | 14 (24.6) | 14 (24.6) | 24 (42.1) | 5 (8.8) | |
| 25 - 36 | 34 | 2 (5.9) | 9 (26.5) | 18 (52.9) | 5 (14.7) | |
| 37 - 60 | 30 | 0 (0) | 2 (6.7) | 5 (16.7) | 23 (76.7) | |
| Total | 190 | 44 (23.2) | 49 (25.8) | 64 (33.7) | 33 (17.3) | |
The highest (33.3%, n = 18) and lowest (18.5%, n = 10) positive RV ELISA results were for infants with mixed and exclusively breastfeeding or varied nutrition, respectively.
4.3. RV Status and Symptoms
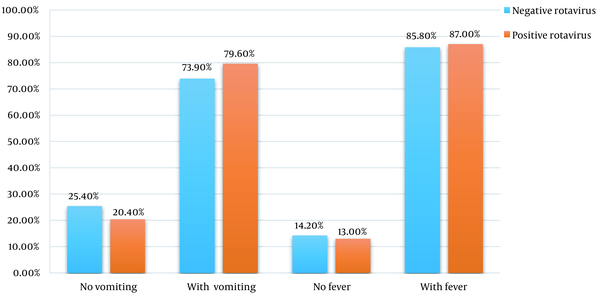
Based on the findings, in the last 4 weeks, fever and cough were reported in 82.3% (n = 158) and 18.8% (n = 36) of hospitalized children (n = 190), respectively. At admission, 86.3% (n = 164) had a fever. In other words, 85.8% (n = 115) of the RV– group (n = 134) and 87.03% (n = 47) of the RV+ group (n = 54) had a fever. There was no significant difference between the prevalence of fever at admission in the positive and negative RV ELISA groups.
Also, 75.7% (n = 144) of hospitalized children had vomiting. It included 73.9% (n = 99) of the RV– group and 79.6% of the RV+ group (n = 43). No significant difference was observed between the 2 groups. The comparison of symptoms at admission between the 2 groups is shown in Figure 1. The average number of diarrhea episodes in 24 hours was 5 to 6 times.
The symptoms at admission in rotavirus positive vs. rotavirus negative
4.4. RV Status and Seasons
The distribution of RV outbreaks in different seasons is shown in Table 3. The number of hospitalized children with diarrhea and RV-positive was highest in winter 2020 (38.90%) and lowest (5.60%) in summer. The prevalence of RV was similar in spring and autumn (22.20%). The highest prevalence of RV– was in autumn (39.60%) and lowest in summer. In terms of the prevalence of diarrhea in seasons, a significant difference was observed between the 2 groups (P = 0.006).
The Rotavirus Enzyme-Linked Immunosorbent Assay and Seasons
| Variable | Season (%) | P Value a | ||||
|---|---|---|---|---|---|---|
| Winter 2020 | Spring 2021 | Summer 2021 | Autumn 2021 | Winter 2021 | ||
| RV ELISA result | 0.006 | |||||
| Negative | 12.70 | 26.90 | 14.20 | 39.60 | 6.70 | |
| Positive | 38.90 | 22.20 | 5.60 | 22.20 | 11.10 | |
4.5. Treatment of RV
The findings showed that 13.5% of children received cefixime before hospitalization, and 40% received antibiotics at the hospital, most of which were ceftriaxone. At the visit, there was moderate to severe dehydration in 93% of children.
4.6. RV ELISA Results and Outcome Status
In the RV– group, 98.5% (n = 132) were discharged with complete recovery, and 1.5% died by other causes than diarrhea. None of the diarrhea caused by RV infection resulted in death. All children in the RV+ group (n = 54) were recovered and discharged.
5. Discussion
This study aimed to determine the frequency of RV with diarrhea in hospitalized children ≤ 5 years old admitted to Mofid Children’s Hospital from December 2020 to March 2021.
The findings of this study showed that the overall prevalence of RV infection was higher in boys (especially those aged ≤ 12 months old) and children with mixed feeding. The lowest frequency of RV-positive was in children with exclusive breastfeeding and varied nutrition. Regarding nutrition status, a significant difference was found between the groups. The highest prevalence of RV-positive was in the winter of 2020, and the lowest was in the summer of 2021. There were significant differences between RV-positive and RV-negative prevalence rates in different seasons of the year (P = 0.006). The fever rate in the RV-positive group was not significantly different from the RV-negative group. Also, the prevalence of vomiting in the RV-positive group did not show a significant difference from the RV-negative group. Further, 88.8% of children with RV-positive had non-exudative stools, and 11.3% had exudative stools, which seems not to be significantly different between the groups (P = 0.07). This study showed that all children with RV-positive recovered and were discharged from the hospital.
The prevalence of RV infection was 28.2%, which is almost similar to the findings in Baghdad City, Iraq (30.3%) (9), Ramadi City, Iraq (32%) (3), Kaduna State, Nigeria (32.2%) (10), Ramadi City, Iraq (32.6%) (6), and Nigeria (37.1%) (11), higher than some findings in china (20.8%) (12), India (18.0%) (1), and Kenya (14.5%) (13), and lower than the study of Habib et al. in Karachi, India (63%) (7). It is necessary to mention that this study was performed during the COVID-19 pandemic when compliance with health issues was more, and as a result, the spread of infectious diseases was less. This may be the reason for the lower prevalence of RV in Iran compared to the mentioned countries. Also, the difference in these results can be due to different diagnosis RV tests, such as ELISA (3, 10-13), multiplex real-time polymerase chain reaction (PCR) (6), and rapid stool antigen immune-chromatographic (ICT) test (1). The RV-PCR seems to be 16 times more sensitive than ELISA (14) and can mask true prevalence changes (15). However, in the reviewed researchers in the current study, the results of PCR were observed to be lower than those of ELISA, which is inconsistent with the studies by Arakaki et al. (15) and Kim and Kim (14). Also, the time of the study (July and August 2016) (1) and other techniques (such as a direct interview with parents used to detect gastroenteritis RV) may affect the prevalence estimates (9). Some authors also suggested performing ELISA in addition to PCR (1), which showed a higher prevalence rate (7). In Iran, studies have shown different RV prevalence rates. According to a systematic review examined the prevalence of RV for 30 years in Iran, the mean prevalence rate of RV was 39.9%, and 50% of Iranian cities were infected with it, which seems to be much higher than the current study estimates (16); however, in Birjand, Ilam, and Tehran provinces, it was 6.4%, 16.5%, and 79.3%, respectively (16, 17). These different results could be due to different age groups, study populations, study designs, geographical regions, diagnostic methods, definitions of symptoms, study types, and times of the studies (15, 18). These factors are effective between 5% and 10% of the reported prevalence rates (15).
The findings of this study show that the prevalence of RV infection is higher in boys than in girls. This result is in line with previous studies (3, 6, 7, 9, 10, 13, 17). However, in some of these studies, the difference was not statistically significant (3, 7, 9, 10, 13). Based on these studies, it seems that boys need more clinical care compared to girls. The findings of Uzoma et al. (11) are inconsistent with other studies, showing that the prevalence is slightly higher in girls than in boys.
This study showed that children aged ≤ 12 months were mostly positive for RV, which is consistent with some studies (3, 6, 7, 11). In the studies of Ayyed et al. (3) and Habib et al. (7), the prevalence of RV-positive was reported to be the highest in 6 - 12 months, followed by ≤ 6 months. The higher incidence of RV-positive in 6 - 12 months may be due to the start of complementary food or contaminated water, decreased passive immunity from the mother in the second half of the first year, lack or decrease of breastfeeding, and early complementary feeding, and contaminated water in ≤ 6 months (3). According to the WHO Scientific Working Group report in 1980, most cases of RV are in the age of 6-24 months, and the highest prevalence was at 9 - 12 months (19). In contrast to the above studies, some studies showed the highest prevalence in 12 - 24 (12), 17 - 24 (13), and 25 - 36 months (10), respectively; however, there is no significant relationship between RV-positive and age groups (P > 0.05) (12, 13). Tian et al. showed that the prevalence of RV-positive was lowest in ≤ 6 months, probably due to maternal antibodies in the child’s body and little exposure to the outside, and the highest was in 1 - 2 years, followed by 6 - 11 months; however, there is no significant difference between the groups regarding the gender group (P = 0.422) (12). The difference in RV-positive prevalence by the age group may be due to diagnostic methods.
In this study, the prevalence of RV-positive was the lowest in breastfed infants, which agrees with some studies (3, 10, 11). However, no significant relationship was observed between exclusive breastfeeding and the prevalence of RV-positive (10, 11), except for Ayyed et al. (P < 0.001) (3). In contrast to this study, Muendo et al. showed that the prevalence of RV-positive was more in breastfed children, and there was a weak association between RV infection and the duration of exclusive breastfeeding. In breastfed infants for 6 months or longer, the risk of RV-positive was 1.4 times higher (13). Based on a meta-analysis, it might be no direct correlation between breastfeeding and RV diarrhea (20); however, it is possible that breastfeeding reduces the incidence of gastroenteritis in young children (21) due to antibodies in the mother’s milk (13).
In this study, a significant difference was observed between the prevalence of RV diarrhea and other causes in different seasons, and RV was highest in winter. It is consistent with Tian et al. in china that RV prevalence is common in November, December, and January (12); in contrast, in a study, the highest prevalence was reported in summer, followed by spring and autumn, respectively, and no cases were reported in winter (17). However, a study showed that with every 1°C increase in temperature, the prevalence of RV decreased by 10% (22). It seems the level of development of the country is a stronger predictor compared to the geographical location. In middle- and high-income countries, the prevalence of RV is more seasonal. However, in low- and middle-income countries, different seasonal patterns of disease outbreaks are observed despite similar climates, geographical locations, and levels of development. It seems no single explanation can be given for the change in seasonality of RV disease (23).
The present study showed that fever, vomiting, and non-exudative stools are more common in RV-positive children than in RV-negative ones, but the difference is not significant. These results are consistent with Habib et al. (7). The main cause of diarrheal diseases in the world, especially in developing countries, is RVs. RV infection typically begins with the sudden onset of diarrhea and vomiting, which sometimes leads to dehydration. Fever is present in most patients. Almost all children get this infection in the first 3 to 5 years of life, but severe diarrhea and dehydration are usually seen between the ages of 3 and 35 months (7). Almost all children with RV infection experience vomiting with 1 to 4 episodes per day (13). The current study is consistent with this finding.
Although the results of the present study are consistent with many studies, the interpretation of the findings should be made with caution because most of the findings obtained were not statistically significant. It also had limitations, including a small sample size, hospital-based study, lack of examination of outpatients, lack of supplementary nutrition status information, education and socio-economic status of parents, and using one method to diagnose a disease. It is suggested to use a larger sample size, a population-based study, and other diagnostic methods (such as PCR) to more accurately estimate the prevalence of the disease and its related variables. Also, measures such as the national registry of RV infection and immunization through RV vaccination are recommended.
5.1. Conclusion
RV infection is prevalent in about one-third of hospitalized children with diarrhea. RV is more common in boys (especially those under 1 year) than in girls. It is lowest in breastfed children. It is also common in winter. There is no significant difference between them, and in many cases, it leads to severe dehydration. This issue requires the implementation of vaccination against RV in the country.
References
-
1.
Manick Dass S, Pattnaik S, Amulya K. A study on prevalence of rotavirus infection in children below 5 years, with acute gastroenteritis. Int J Community Med Public Health. 2018;5(8):3358. https://doi.org/10.18203/2394-6040.ijcmph20183061.
-
2.
World Health Organization. Rotavirus. Geneva, Switzerland: World Health Organization; 2022, [cited 2022]. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/rotavirus.
-
3.
Ayyed M, Al-Dulaimi MF, Al-Ani RK, Al-Mawla SO. Incidence of Rota Virus Gastroenteritis among Vaccinated and Non-vaccinated Children Less Than Two Years Old in Ramadi City, Iraq. Al-Anbar Medical Journal. 2020;16(1). https://doi.org/10.33091/amj.0601522019.
-
4.
Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000-2013. Clin Infect Dis. 2016;62 Suppl 2:S96-s105. [PubMed ID: 27059362]. https://doi.org/10.1093/cid/civ1013.
-
5.
Shoja Z, Jalilvand S, Mollaei-Kandelous Y, Validi M. Epidemiology of viral gastroenteritis in Iran. Pediatr Infect Dis J. 2014;33(2):218-20. [PubMed ID: 23989109]. https://doi.org/10.1097/inf.0000000000000028.
-
6.
Abd-Al Fattah SA, Hamad MA, AL-Ani MQ. Prevalence and Molecular Detection of Rotavirus in Children in Ramadi City-Iraq. Medico Legal Update. 2020;20(4):2096-102.
-
7.
Habib MI, Kazi SG, Ahmed Khan KM, Zia N. Rota virus Diarrhea in Hospitalized Children. J Coll Physicians Surg Pak. 2014;24(2):114-7. [PubMed ID: 24491006].
-
8.
Getachew HB, Dahl RM, Lopman BA, Parashar UD. Rotavirus Vaccines and Health Care Utilization for Diarrhea in US Children, 2001 to 2015. Pediatr Infect Dis J. 2018;37(9):943-8. [PubMed ID: 29561514]. [PubMed Central ID: PMCPmc7147954]. https://doi.org/10.1097/inf.0000000000001988.
-
9.
Hussein SSM, Fadhil SA, Salman RY. Efficacy of Rotavirus Vaccination in Prevention of Hospital Admission of Gastroenteritis among Children Under Two Years of Age. Iraqi Postgraduate Medical Journal. 2018;17(3):225-31.
-
10.
Mohammed AA, Aminu M, Ado SA, Jatau ED, Esona MD. Prevalence of rotavirus among children under five years of age with diarrhea in Kaduna State, Nigeria. Niger J Paediatr. 2016;43(4):264. https://doi.org/10.4314/njp.v43i4.6.
-
11.
Uzoma EB, Chukwubuikem C, Omoyibo E, Tagbo O. Rota virus genotypes and the clinical severity of Diarrhoea among children under 5 years of age. Niger Postgrad Med J. 2016;23(1):1-5. [PubMed ID: 27098941]. https://doi.org/10.4103/1117-1936.180108.
-
12.
Tian Y, Chughtai AA, Gao Z, Yan H, Chen Y, Liu B, et al. Prevalence and genotypes of group A rotavirus among outpatient children under five years old with diarrhea in Beijing, China, 2011-2016. BMC Infect Dis. 2018;18(1):497. [PubMed ID: 30285635]. [PubMed Central ID: PMCPmc6168998]. https://doi.org/10.1186/s12879-018-3411-3.
-
13.
Muendo C, Laving A, Kumar R, Osano B, Egondi T, Njuguna P. Prevalence of rotavirus infection among children with acute diarrhoea after rotavirus vaccine introduction in Kenya, a hospital cross-sectional study. BMC Pediatr. 2018;18(1):323. [PubMed ID: 30309343]. [PubMed Central ID: PMCPmc6180366]. https://doi.org/10.1186/s12887-018-1291-8.
-
14.
Kim HS, Kim JS. Discrepancies between Antigen and Polymerase Chain Reaction Tests for the Detection of Rotavirus and Norovirus. Ann Clin Lab Sci. 2016;46(3):282-5. [PubMed ID: 27312553].
-
15.
Arakaki L, Tollefson D, Kharono B, Drain PK. Prevalence of rotavirus among older children and adults with diarrhea: A systematic review and meta-analysis. Vaccine. 2021;39(33):4577-90. [PubMed ID: 34244008]. https://doi.org/10.1016/j.vaccine.2021.06.073.
-
16.
Monavari SHR, Hadifar S, Mostafaei S, Miri A, Keshavarz M, Babaei F, et al. Epidemiology of Rotavirus in the Iranian Children: A Systematic Review and Meta-analysis. J Glob Infect Dis. 2017;9(2):66-72. [PubMed ID: 28584458]. [PubMed Central ID: PMCPmc5452554]. https://doi.org/10.4103/0974-777x.205173.
-
17.
Mohammadi J, Amini R, Akbari A, Amraei M, Mahmoudvand S, Jalilian FA. Prevalence and Seasonal Frequency of acute viral gastroenteritis in children less than 5 years in Ilam, Iran. Prevalence. 2020;7(3):66-74.
-
18.
Luchs A, Cilli A, Morillo SG, de Cassia Compagnoli Carmona R, do Carmo Sampaio Tavares Timenetsky M. Rotavirus in adults, Brazil, 2004-2011: G2P[4] dominance and potential impact on vaccination. Braz J Infect Dis. 2014;18(1):53-9. [PubMed ID: 24076114]. [PubMed Central ID: PMCPmc9425225]. https://doi.org/10.1016/j.bjid.2013.05.010.
-
19.
WHO Scientific Working Group. Rotavirus and other viral diarrhoeas: WHO scientific working group. Bull World Health Organ. 1980;58(2):183-98. [PubMed ID: 6249509]. [PubMed Central ID: PMCPmc2395787].
-
20.
Shen J, Zhang BM, Zhu SG, Chen JJ. No direct correlation between rotavirus diarrhea and breast feeding: A meta-analysis. Pediatr Neonatol. 2018;59(2):129-35. [PubMed ID: 28958831]. https://doi.org/10.1016/j.pedneo.2017.06.002.
-
21.
Hartman S, Brown E, Loomis E, Russell HA. Gastroenteritis in Children. Am Fam Physician. 2019;99(3):159-65. [PubMed ID: 30702253].
-
22.
Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38(6):1487-96. [PubMed ID: 19056806]. [PubMed Central ID: PMCPmc2800782]. https://doi.org/10.1093/ije/dyn260.
-
23.
Patel MM, Pitzer VE, Alonso WJ, Vera D, Lopman B, Tate J, et al. Global seasonality of rotavirus disease. Pediatr Infect Dis J. 2013;32(4):e134-47. [PubMed ID: 23190782]. [PubMed Central ID: PMCPmc4103797]. https://doi.org/10.1097/INF.0b013e31827d3b68.

