Abstract
Background:
Nosocomial infections constitute a global health problem, leading to a high rate of morbidity and mortality. The choice of antimicrobial treatment for nosocomial infections is often empirical and based on the knowledge of local antimicrobial activity patterns of the most common bacteria causing such infections.Objectives:
The aim of this study was to determine the 3 most prevalent bacterial pathogens including Acinetobacter baumannii, Pseudomonas aeruginosa and Staphylococcus aureus causing nosocomial infections and their antimicrobial resistant profiles in patients admitted to three hospitals in Tehran city, Iran.Materials and Methods:
In this cross-sectional study, the A. baumannii, P. aeruginosa and S. aureus isolates were obtained from different samples of patients with nosocomial infections admitted to different wards of three hospitals including Milad, Motahary and Loghman from November 2014 to April 2015. Nosocomial infections were defined as a culture-proven infection, which occurred more than 48 hours after admission. Antimicrobial susceptibility testing was performed using the disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines.Results:
In total, 539 samples were collected during the study period from patients with nosocomial infections. Overall, 198, 75 and 98 A. baumannii, P. aeruginosa and S. aureus isolates were obtained, respectively. Cefepim and meropenem were found to be the most effective antibiotics for nosocomial infections caused by S. aureus with only 1 resistant isolate. Resistance to gentamicin and amikacin and susceptibility to cefepim was the highest compared to other antibiotics amongst P. aeruginosa isolates which is in consistent with the fact that cephalosporins remain useful agents for the management of nosocomial infections caused by P. aeruginosa. Acinetobacter baumannii isolates showed lower susceptibility rates to imipenem and ciprofloxacin than other antibiotics with 189 and 187 resistant isolates, respectively; however 155 isolates were susceptible to co-trimoxazole and ceftazidime. The high resistant rate amongst A. baumannii to carbapenems is likely consequence of heavy empirical usage of this group of antibiotics.Conclusions:
The high prevalence of antimicrobial resistance in the hospitals highlights the need for further infection control programs. Future regional epidemiological data on antimicrobial resistance patterns will be required to implement strict national antibiotic policies to restrict the spread of these resistance bugs.Keywords
Nosocomial Infection A. baumannii P. aeruginosa S. aureus Antimicrobial Drug Resistance
1. Background
Nosocomial infections have been described as one of the common complications of hospitalized patients. Nosocomial infections can cause increased patient morbidity, affect the success of initial illness treatment that the patient is hospitalized for, delay patient discharge which then causes additional costs for the health care system and even result in patient death (1).
Development and implementation of proper infection control programs to prevent spread of antimicrobial resistant bacteria through clinics and hospitals have been shown to be a key component in reducing infection rates (2); however, nosocomial infections cannot be completely eliminated. In spite of bacterial culture and antimicrobial susceptibility testing for diagnosis of bacterial growth and to guide best antibiotic treatment, empirical treatment is often necessary (3).
Acinetobacter baumannii has emerged worldwide as important pathogenic bacteria causing nosocomial infections due to the emergence of multidrug resistant (MDR) A. baumannii (4). The nosocomial infections surveillance of our hospitals showed that A. baumannii infections have increased in our hospitals since the last 5 years, during which time MDR A. baumannii were mainly isolated from patients in each hospital.
Pseudomonas aeruginosa is a major nosocomial pathogen, which survives in moist environments and colonizes the respiratory tract of mechanically ventilated patients (5). It causes severe infections such as pneumonia in critically ill and immunocompromised patients (6). Multidrug resistant P. aeruginosa is especially associated with increased mortality because no adequate therapeutic option exists. Recently, MDR P. aeruginosa has been variously reported worldwide (7, 8).
Staphylococcus aureus can also be a significant pathogen, causing surgical wound infection, bacteremia, endocarditis, osteomyelitis and pneumonia. Staphylococcus aureus colonization may occur long before or immediately before infection, but either way, it plays a major role in the development of nosocomial infections (9). The most common species causing nosocomial infections are Methicillin-Resistant Staphylococcus aureus (MRSA). Importance of MRSAs as a pathogen has increased with the occurrence of high-level resistance to multiple antimicrobial drugs, such as co-amoxiclav, cefotaxime and gentamicin (10, 11).
Therefore, appropriate and effective antibiotic treatment is a growing concern in hospitals as an increase in MDR pathogens, especially the emergence of MDR clones of A. baumannii, P. aeruginosa and S. aureus, is reported.
2. Objectives
The aim of this study was to estimate the distribution of A. baumannii, P. aeruginosa and S. aureus isolates collected from hospitalized patients with nosocomial infections and to assess susceptibility patterns to clinically relevant antimicrobials.
3. Materials and Methods
Three referral hospitals including Milad, Motahary and Loghman hospitals were enrolled in the study. In this cross-sectional study, all the A. baumannii, P. aeruginosa and S. aureus isolates were obtained from different samples of patients with nosocomial infections admitted to surgery, neurosurgery, internal diseases, intensive care unit (ICU) and post coronary care unit (CCU) wards of each hospital between November 2014 and April 2015. Nosocomial infections were defined as a culture-proven infection, which occurred more than 48 hours after admission. Samples were obtained as soon as nosocomial infection was suspected. Patients were given adequate and efficient antibiotic regimen immediately after sampling.
The bacterial strains were obtained from urine, stool and sputum samples, nasopharyngeal swabs and blood cultures. The obtained samples were inoculated and cultured on agar-based culture media on the day of arrival. All inoculated agar plates were incubated at 37°C for 24 to 48 hours until the presence of adequate growth. Identification of pathogens was based on colony type and morphology, Gram-staining characteristics and standard biochemical tests.
The antimicrobial susceptibility testing was performed using the disk diffusion method, according to the clinical and laboratory standard institute (CLSI) guidelines (12). The Muller-Hinton agar was used uniformly for all the sensitivity testing and incubated aerobically at 37°C. Escherichia coli ATCC 25922 was used as a quality control strain.
The antibiotics for disk diffusion testing were selected according to the CLSI guidelines; these antibiotics were chosen because they are representative of their antibiotic classes and their Minimum Inhibitory Concentrations (MICs) were tested throughout the study period. An isolate was classified as MDR if it was resistant to three or more antimicrobial classes to which that bacterial species are normally susceptible.
All statistical analyses were performed using SPSS 15.0 (USA) software.
4. Results
4.1. Species Distribution
In total, 539 samples were collected during the study period from patients with nosocomial infections who were without antimicrobial exposure in the last 12 weeks and their average hospital stay was 1 month.
Overall, 530 positive specimens were collected from which 198, 75 and 98 A. baumannii, P. aeruginosa and S. aureus isolates were obtained respectively. The distribution of the isolates was: 39, 115 and 44 A. baumannii isolates, 4, 41 and 30 P. aeruginosa isolates and 51, 34 and 13 S. aureus isolates from Loghman, Milad and Motahary hospitals, respectively.
4.2. Antimicrobial Resistance of Isolates
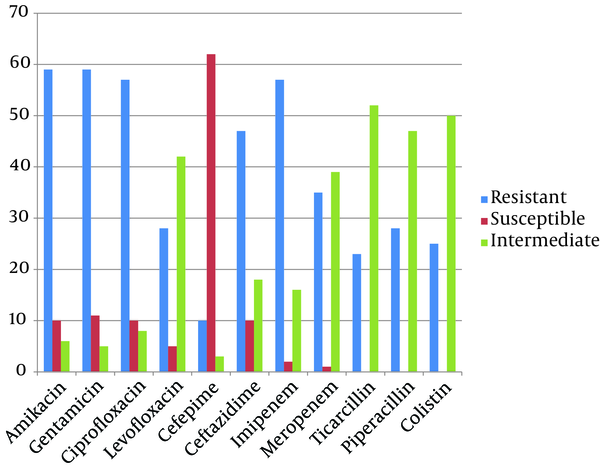
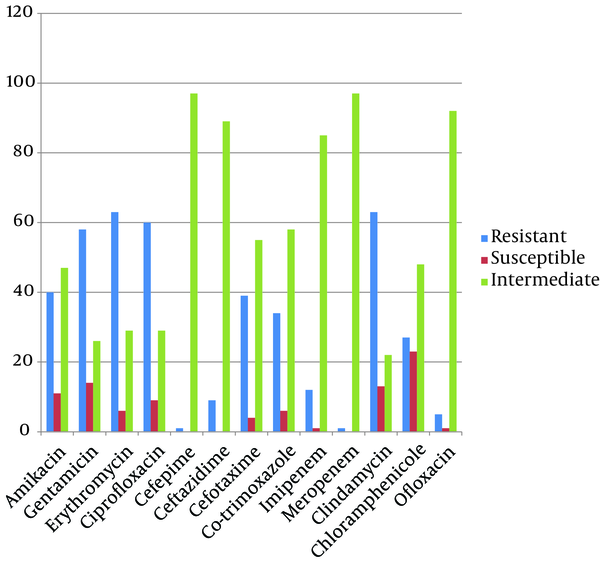
Antimicrobial resistance of A. baumannii, P. aeruginosa and S. aureus isolates are summarized in Figure 1, 2 and 3, respectively. Resistance to imipenem was the highest among A. baumannii isolates; meanwhile these isolates were susceptible to ceftazidime as being the most efficient antibiotic for A. baumannii nosocomial infections treatment (Figure 1). The highest resistance among P. aeruginosa isolates was observed to gentamicin and amikacin and they were most susceptible to cefepim (Figure 2). We found cefepim and meropenem to be the best antibiotic of choice for the treatment of S. aureus nosocomial infections; however, clindamycin seems to have no efficacy on S. aureus infections due to high resistance observed among these isolates (Figure 3). Moreover, 67, 26 and 20 (total: 113) A. baumannii, 16, 7 and 2 P. aeruginosa (total: 25) and 9, 1 and 5 S. aureus isolates (total: 15) obtained from Loghman, Milad and Motahary hospitals, respectively, were found to be MDR.
Antibiotic Resistance Pattern of Pseudomonas aeruginosa Isolates by Disk diffusion Method From Patients With Nosocomial Infections in Loghman, Milad and Motahary Hospitals
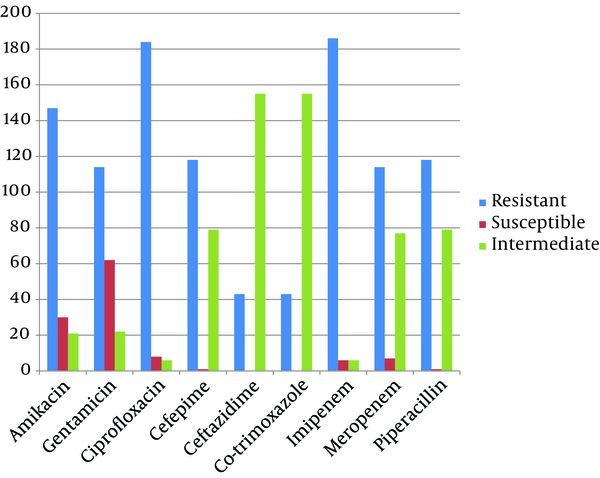
Antibiotic Resistance Pattern of Acinetobacter baumannii Isolates by Disk Diffusion Method From Patients With Nosocomial Infections in Loghman, Milad and Motahary Hospitals
Antibiotic Resistance Pattern of Staphylococcus aureus Isolates by Disk Diffusion Method From Patients With Nosocomial Infections in Loghman, Milad and Motahary Hospitals
Definition for resistance, susceptible and intermediate isolates was as described by CLSI for different antibiotics (12).
5. Discussion
The emergence of resistant bacteria causing nosocomial infections has become a health care issue that has caused serious concern to physicians (13). The increase of invasive nosocomial infections caused by multi-resistant bacteria, however, did not only increase the total burden of nosocomial infections, but also resulted in a much more complicated status of the hospitalized patients (14). This study investigated the prevalence and antibacterial resistance patterns of three health-threatening bacteria including A. baumannii, P. aeruginosa and S. aureus isolated from patients admitted to hospital wards suffering from nosocomial infections. The number of isolates causing nosocomial infections in Milad hospital was found to be higher in comparison with Loghman and Motahary hospitals, which may be as a result of higher admission rates in this hospital.
Acinetobacter baumannii is one of the most common pathogenic bacteria causing nosocomial infections especially in ICUs. Also, MDR A. baumannii is the main pathogenic bacteria in the ICUs (15, 16). Immunodeficient patients in ICUs are much more susceptible to A. baumannii infection since ICUs are prone to contamination and thereby spreading pathogens to patients (17, 18). A survey on the patients with A. baumannii infection revealed that most strains were isolated from patients in ICUs and these patients manifested symptoms of respiratory tract infection, suggesting that MDR A. baumannii mainly causes respiratory infections in the hospitals and infects patients in ICUs (19). This bacterium also infects patients who undergo invasive operations (4).
Pseudomonas aeruginosa was maximally isolated from the samples of patients admitted to the neurosurgery wards followed by neurosurgery and surgery ICUs, surgery wards and orthopedic wards suggesting the fact that nosocomial infections due to P. aeruginosa were seen more in the postoperative patients and those who are in the hospital for a long time (20). This finding is in consistent with a retrospective case-control study in Turkey and a retrospective cross-sectional study in India which concluded that the major risk factors for infection or colonization with multi-resistant P. aeruginosa were prolonged stay in the ICU (20, 21). Of the total P. aeruginosa isolates, maximum MDR isolates were obtained from the wards mentioned above, respectively. Previous studies demonstrated that patients with nosocomial infections caused by P. aeruginosa can be divided into 3 groups: patients for whom their treatment had to be revised but later discharged healthy; patients who required no extra interventions in the treatment protocol and the infection was controlled simply by removing the offending implant/devices or after minor debridement; patients who died due to P. aeruginosa nosocomial infection (20, 22, 23). Furthermore, it was seen that P. aeruginosa mostly colonizes on the patients surrounding area and instruments and thus, spreading to the patients; hence, simple cleaning was able to minimize the infection rate considerably.
Staphylococcus aureus is a part of human skin normal flora; however, since aseptic preparation of the skin cannot completely eliminate skin-associated bacteria, especially not bacteria residing in the deeper parts of the skin such as the hair follicles and sebaceous glands, S. aureus is globally reported as an important cause of clinical infections including nosocomial infections (24). The risk of nosocomial infections with S. aureus has been highlighted lately, particularly with the emergence of multi-resistant clones such as MRSA (25). Most of the S. aureus isolates were obtained from patients underwent surgical procedures, which is in consistent with the fact that bacteria can enter deeper tissues during the initial incision.
The A. baumannii isolates possess an intrinsically high resistant to imipenem (95.5%) and ciprofloxacin (94.5%). Furthermore, A. baumannii is less susceptible to cephalosporin agents because their Penicillin-Binding Proteins (PBPs) have lower affinities for these antibiotics and some strains have plasmid-encoded β-lactamases. In our study, the relatively high resistance rate to amikacin in A. baumannii isolates was higher than the resistance rates reported by Khelatabadi et al. (26) in Iran and Wang et al. (27) in Taiwan and Smolyakov et al. (28) in Israel. Fazeli et al. found ampicillin-sulbactam to be the most efficient antibiotic for nosocomial infections caused by A. baumannii with only 33.9% resistance isolates (16).The resistance rate to meropenem was observed in 57.5% of A. baumannii isolates which is about 40% higher than resistance rates reported by Feizabadi et al. (29) and Mirnejad et al. (30) in Iran and Karlowsky et al. (31) in United states, Basustaoglu et al. (32) in Turkey and Hujer et al. (33) in United states. The high resistance rate to β-lactam antibiotics might be due to a wide usage of these antimicrobial agents in Tehran hospital wards in the last few years.
Fluoroquinolones are frequently used with or without cell-wall-active antibiotics for P. aeruginosa infections (34). However, prior fluoroquinolone use has been identified as a risk factor for the emergence of imipenem/meropenem resistant P. aeruginosa (20, 34). Out of 57 (76%) imipenem/meropenem-resistant P. aeruginosa isolated during the study period, 43 (57%) showed resistance to levofloxacin and ciprofloxacin, which is in consistent with the fact that cross-resistance may have developed for imipenem/meropenem due to prior use of fluoroquinolones. In a study by Livermore, cross-resistance to fluoroquinolones, cephalosporins, aminoglycosides and β-lactam antibiotics is reported, as well (35). In spite of the fact that many studies have reported high resistance to cephalosporins among P. aeruginosa isolates (36, 37), based on the current study, cephalosporins remain efficient antibiotics in P. aeruginosa infections, concerning the most susceptibility to cefepim with only 10 (13%) resistant isolates; however, colistin was also a promising antimicrobial agent in P. aeruginosa infections with 29% resistance rate. In a research on P. aeruginosa isolates obtained from patients with burn wounds, colistin was investigated to be the only efficient antimicrobial agent (38). Further studies seem essential to determine the antimicrobial agent with less resistant isolates for treatment of P. aeruginosa infections.
Methicillin-resistant Staphylococcus aureus are among the most frequent species responsible for nosocomial infections worldwide. Japoni et al. (10) in 2004 in Iran, Udo et al. (11) in 2008 in Kuwait and Madani et al. (24) in 2001 in Saudi Arabia found MRSA species to be the most prevalent S. aureus species causing nosocomial infections. In the present study, high resistance to clindamycin (64%) and erythromycin (64%) were observed among the S. aureus isolates; these results are comparable to resistance rates reported by Fatholahzadeh et al. in Iran (39), which means that these two antibiotics are no longer reliable agents in the treatment of S. aureus infections. Due to low susceptibility to aminoglycosides and fluoroquinolones (except for ofloxacin) as well as clindamycin and erythromycin, controlling the spread of these organisms has become of paramount importance.
The high prevalence of concomitant resistance to different antimicrobial agents has resulted in attempts made to look for alternative antibiotics in several studies. Fluoroquinolones and β-lactam antibiotics have been among the dominant class of antimicrobial agents widely used for nosocomial infections empirically in the last decade (3). In our study, relatively high resistance to fluoroquinolones and β-lactam antibiotics among all 3 bacterial species was observed and thus, these widely used classes of antimicrobial agents for empirical treatment of nosocomial infections caused by A. baumannii, P. aeruginosa and S. aureus could not be effective. It is worth to mention that the number of MDR isolates was higher among isolates collected from Loghman hospital which may be due to extensive and inappropriate use of different antibiotic classes in this hospital wards. All of these, highlight the importance of understanding the status of drug-resistance and local specificities in MDR isolates for antimicrobial empirical therapy, particularly in general hospitals with a higher prevalence of nosocomial infections.
Acknowledgements
References
-
1.
Hong DJ, Bae IK, Jang IH, Jeong SH, Kang HK, Lee K. Epidemiology and Characteristics of Metallo-beta-Lactamase-Producing Pseudomonas aeruginosa. Infect Chemother. 2015;47(2):81-97. [PubMed ID: 26157586]. https://doi.org/10.3947/ic.2015.47.2.81.
-
2.
Jiang MJ. Drug resistance and distribution in common clini¬cal Gram-negative bacilli collected from intensive care units (in Chi¬nese). Chin J Exp Clin Infect Dis. 2012;6:42-5.
-
3.
Abamecha A, Wondafrash B, Abdissa A. Antimicrobial resistance profile of Enterococcus species isolated from intestinal tracts of hospitalized patients in Jimma, Ethiopia. BMC Res Notes. 2015;8:213. [PubMed ID: 26036911]. https://doi.org/10.1186/s13104-015-1200-2.
-
4.
Jiang M, Zhang Z, Zhao S. Epidemiological characteristics and drug resistance analysis of multidrug-resistant Acinetobacter baumannii in a China hospital at a certain time. Pol J Microbiol. 2014;63(3):275-81. [PubMed ID: 25546937].
-
5.
Rossolini GM, Mantengoli E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin Microbiol Infect. 2005;11 Suppl 4:17-32. [PubMed ID: 15953020]. https://doi.org/10.1111/j.1469-0691.2005.01161.x.
-
6.
Giske CG, Monnet DL, Cars O, Carmeli Y, ReAct-Action on Antibiotic R. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother. 2008;52(3):813-21. [PubMed ID: 18070961]. https://doi.org/10.1128/AAC.01169-07.
-
7.
Park YS, Lee H, Chin BS, Han SH, Hong SG, Hong SK, et al. Acquisition of extensive drug-resistant Pseudomonas aeruginosa among hospitalized patients: risk factors and resistance mechanisms to carbapenems. J Hosp Infect. 2011;79(1):54-8. [PubMed ID: 21764173]. https://doi.org/10.1016/j.jhin.2011.05.014.
-
8.
Paterson DL. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43 Suppl 2:S43-8. [PubMed ID: 16894514]. https://doi.org/10.1086/504476.
-
9.
Oteo J, Baquero F, Vindel A, Campos J, Spanish members of the European Antimicrobial Resistance Surveillance S. Antibiotic resistance in 3113 blood isolates of Staphylococcus aureus in 40 Spanish hospitals participating in the European Antimicrobial Resistance Surveillance System (2000-2002). J Antimicrob Chemother. 2004;53(6):1033-8. [PubMed ID: 15128722]. https://doi.org/10.1093/jac/dkh214.
-
10.
Japoni A, Alborzi A, Rasouli M, Pourabbas B. Modified DNA extraction for rapid PCR detection of methicillin resistant staphylococci. Iran Biomed J. 2004;8(3):161-5.
-
11.
Udo EE, Al-Sweih N, Dhar R, Dimitrov TS, Mokaddas EM, Johny M, et al. Surveillance of antibacterial resistance in Staphylococcus aureus isolated in Kuwaiti hospitals. Med Princ Pract. 2008;17(1):71-5. [PubMed ID: 18059105]. https://doi.org/10.1159/000109594.
-
12.
CLSI: Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 21th informational supplement. Wayne: CLSI; 2011. Available from: http://antimicrobianos.com.ar/ATB/wp-content/uploads/2012/11/M100S22E.pdf.
-
13.
Arias CA, Murray BE. Emergence and management of drug-resistant enterococcal infections. Expert Rev Anti Infect Ther. 2008;6(5):637-55. [PubMed ID: 18847403]. https://doi.org/10.1586/14787210.6.5.637.
-
14.
Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9(2):148-65. [PubMed ID: 8964033].
-
15.
Zhao SP, Jiang MJ, Wang GM. Distribution and Anti¬microbial Resistance of Commonly Encountered Gram-negative Bacilli in Clinical in Taian region (in Chinese). Chin J Clin. 2011:179-81.
-
16.
Fazeli H, Taraghian A, Kamali R, Poursina F, Nasr Esfahani B, Moghim S. Molecular Identification and Antimicrobial Resistance Profile of Acinetobacter baumannii Isolated From Nosocomial Infections of a Teaching Hospital in Isfahan, Iran. Avicenna J Clin Microbiol Infect. 2014;1(3). https://doi.org/10.17795/ajcmi-21489.
-
17.
Wang YM, Shen JL. Primary study on efflux pump mechanism in multidrug-resistant Acinetobacter baumannii (in Chi¬nese). Acta Universitatis Medicinalis Anhui. 2012;47:38-40.
-
18.
Shen JL, Zhu DM, Wang MG. The relationship between acquired carbapenemases and resistance of gram-negative bacilli (in Chinese). Chin J Lab Med. 2008;31:408-14.
-
19.
Tang XL, Yang J. Analysis of infection status and drug resistance of Acinetobacter baumannii in ICU (in Chinese). Chong Qing Medcine. 2013;42:302-3.
-
20.
Rajkumari N, John NV, Mathur P, Misra MC. Antimicrobial Resistance in Pseudomonas sp. Causing Infections in Trauma Patients: A 6 Year Experience from a South Asian Country. J Glob Infect Dis. 2014;6(4):182-5. [PubMed ID: 25538457]. https://doi.org/10.4103/0974-777X.145250.
-
21.
Gulay Z, Atay T, Amyes SG. Clonal spread of imipenem-resistant Pseudomonas aeruginosa in the intensive care unit of a Turkish hospital. J Chemother. 2001;13(5):546-54. [PubMed ID: 11760220]. https://doi.org/10.1179/joc.2001.13.5.546.
-
22.
Mardaneh J, Ahmadi KH, Jahan Sepas A. Determination Antimicrobial Resistance Profile of Pseudomonas Aeruginosa Strains Isolated from Hospitalized Patients in Taleghani Hospital (Ahvaz, Iran) from 2011-2012. J Fasa Univ Med Sci. 2013;3(3):188-93.
-
23.
Tsukayama DT, van Loon HJ, Cartwright C, Chmielewski B, Fluit AC, van der Werken C, et al. The evolution of Pseudomonas aeruginosa during antibiotic rotation in a medical intensive care unit: the RADAR-trial. Int J Antimicrob Agents. 2004;24(4):339-45. [PubMed ID: 15380258]. https://doi.org/10.1016/j.ijantimicag.2004.04.011.
-
24.
Madani TA, Al-Abdullah NA, Al-Sanousi AA, Ghabrah TM, Afandi SZ, Bajunid HA. Methicillin-resistant Staphylococcus aureus in two tertiary-care centers in Jeddah, Saudi Arabia. Infect Control Hosp Epidemiol. 2001;22(4):211-6. [PubMed ID: 11379711]. https://doi.org/10.1086/501891.
-
25.
Hsueh PR, Teng LJ, Chen WH, Pan HJ, Chen ML, Chang SC, et al. Increasing prevalence of methicillin-resistant Staphylococcus aureus causing nosocomial infections at a university hospital in Taiwan from 1986 to 2001. Antimicrob Agents Chemother. 2004;48(4):1361-4. [PubMed ID: 15047544].
-
26.
Farahani Khelatabadi R, Moniri R, Shajari G. Antibiotic resistance patterns and the spread of antibiotic resistance genes in Acinetobacter species isolated from Kashan. Feyz, Kashan Univ Med Sci Health Serv. 2009;12(4):60-6.
-
27.
Wang SH, Sheng WH, Chang YY, Wang LH, Lin HC, Chen ML, et al. Healthcare-associated outbreak due to pan-drug resistant Acinetobacter baumannii in a surgical intensive care unit. J Hospital Infect. 2003;53(2):97-102. https://doi.org/10.1053/jhin.2002.1348.
-
28.
Smolyakov R, Borer A, Riesenberg K, Schlaeffer F, Alkan M, Porath A, et al. Nosocomial multi-drug resistant Acinetobacter baumannii bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment. J Hospital Infect. 2003;54(1):32-8. https://doi.org/10.1016/s0195-6701(03)00046-x.
-
29.
Feizabadi MM, Fathollahzadeh B, Taherikalani M, Rasoolinejad M, Sadeghifard N, Aligholi M, et al. Antimicrobial susceptibility patterns and distribution of blaOXA genes among Acinetobacter spp. Isolated from patients at Tehran hospitals. Jpn J Infect Dis. 2008;61(4):274-8. [PubMed ID: 18653968].
-
30.
Mirnejad R, Dehghani M, Masjedian F, Imani Fooladi A, Haghighat S. Antimicrobial Susceptibility Patterns and Distribution of bla kpc Genes among Acinetobacter baumannii Isolated from Patients at Tehran - Iran Hospitals. J Pure Appl Microbiol. 2012;6(2):1-6.
-
31.
Karlowsky JA, Draghi DC, Jones ME, Thornsberry C, Friedland IR, Sahm DF. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother. 2003;47(5):1681-8. [PubMed ID: 12709340].
-
32.
Basustaoglu AC, Kisa O, Sacilik SC, Ozyurt M, Yildiran ST. Epidemiological characterization of hospital-acquired Acinetobacter baumannii isolates from a 1500-bed teaching hospital by phenotypic and genotypic methods. J Hosp Infect. 2001;47(3):246-7. [PubMed ID: 11247688]. https://doi.org/10.1053/jhin.2000.0867.
-
33.
Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50(12):4114-23. [PubMed ID: 17000742]. https://doi.org/10.1128/AAC.00778-06.
-
34.
Furtado GH, Bergamasco MD, Menezes FG, Marques D, Silva A, Perdiz LB, et al. Imipenem-resistant Pseudomonas aeruginosa infection at a medical-surgical intensive care unit: risk factors and mortality. J Crit Care. 2009;24(4):625 e9-14. [PubMed ID: 19592213]. https://doi.org/10.1016/j.jcrc.2009.03.006.
-
35.
Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34(5):634-40. [PubMed ID: 11823954]. https://doi.org/10.1086/338782.
-
36.
Abdoli Oskouie S, Ahangarzade Rezaie M, Panahi F, Firoozi F, Es.Haghi M, Panahi F. Microbiological evaluation of nosocomial infections by using National Nosocomial Infection Surveillance (NNIS) system. Arch Pediatr Infect Dis. 2014:217-24.
-
37.
Hadadi A, Rasoulinejad M, Zia Bashar Hagh N. Investigation of the frequency of gram negative nosocomial infections and evaluation of antimicrobial resistance patterns by E-test and Disk diffusion methods in Sina Hospital. Trauma Month. 2008;13(1):51-27.
-
38.
Adabi M, Talebi Taher M, Arbabi L. Determination of antibiotic resistance pattern of Pseudomonas aeruginosa strains isolated from patients with burn wounds. J Ardabil Univ Med Sci. 2015;15(1):66-74.
-
39.
Fatholahzadeh B, Emaneini M, Gilbert G, Udo E, Aligholi M, Modarressi MH, et al. Staphylococcal cassette chromosome mec (SCCmec) analysis and antimicrobial susceptibility patterns of methicillin-resistant Staphylococcus aureus (MRSA) isolates in Tehran, Iran. Microb Drug Resist. 2008;14(3):217-20. [PubMed ID: 18694326]. https://doi.org/10.1089/mdr.2008.0822.
