Abstract
Keywords
Septic Arthritis Osteomyelitis Children Streptococcus dysgalactiae
1. Introduction
Streptococcus dysgalactiae subspecies equisimilis (SDSE) is a gram positive bacterium of the beta-haemolytic streptococci group; its further identification is based on Lancefield group carbohydrate antigens (1). In the new taxonomy all large colony-forming groups C and G streptococci are classified as SDSE (2).
The isolation of SDSE in normally sterile sites and its association with a similar spectrum of diseases to that caused by Streptococcus pyogenes (SP) has raised the attention of medical community and is considered an emerging infection (3).
The clinical reports of SDSE infections in the pediatric population are scarce as the identified predisposing factors for invasive infections are limited to old and previously ill subjects
2. Case Presentation
A three-year-old toddler was admitted to clinic with a history of 24 hours of high fever and severe groin pain. She belonged to a gypsy ethnic group with a history of recurrent periodontal phlegmons with decay in several teeth. She had no contact with animals. On physical examination, the left leg was held in a position of slight flexion, abduction and external rotation, with severe pain during movement. She could not achieve a sitting position or bear weight.
Initial laboratory investigations revealed a white cell count of 24,100 with 19,580 (81%) neutrophils and elevated acute phase reactants: C-reactive protein (CRP) 8.3 mg/dL, the erythrocyte sedimentation rate (ESR) 65 mm/hr. Ultrasonographic examination of the left hip revealed an effusion. Septic arthritis of the left hip was suspected and she was treated with intravenous (IV) cefotaxime (200 mg/kg) and cloxacillin (150 mg/kg) after obtaining blood culture results. She underwent surgical drainage of the hip within the first 24 hours after admittance obtaining purulent material from the joint, which was processed for culture.
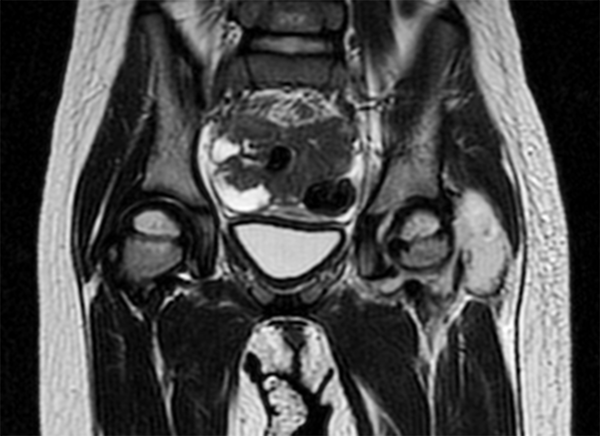
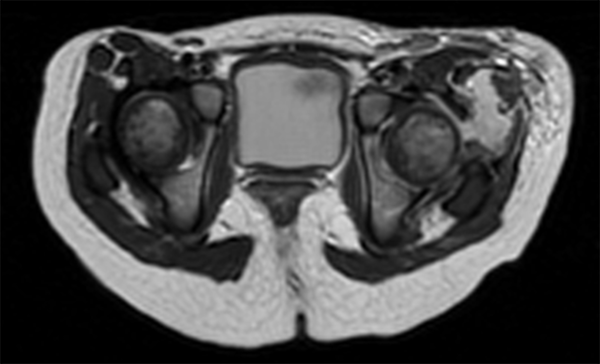
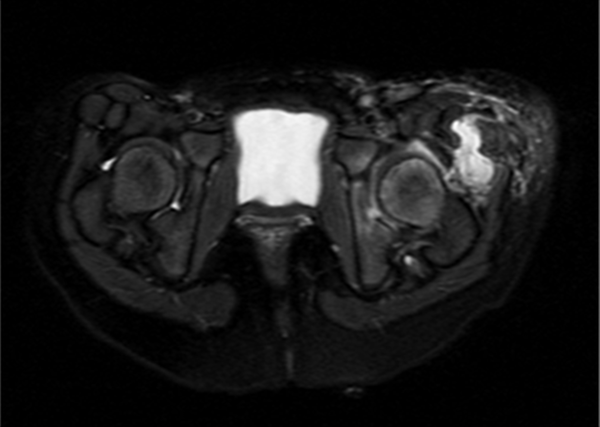
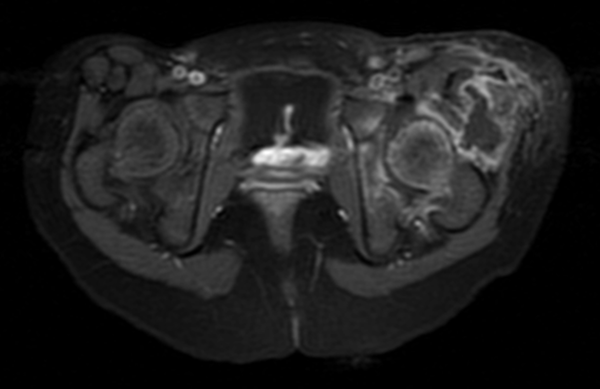
Magnetic resonance imaging (MRI) showed a left hip effusion and edema in femoral head, neck, great and lesser trochanters and acetabulum. T2 sequences showed increased signal in obturator, and gluteus muscles and nearby subcutaneous tissue (Figure 1 - 3). On fat suppression T1 sequence after gadolinium administration (Figure 4), a peripheral enhancement of the periarticular collection and a low signal zone in the femoral head appeared, suggesting abscessification in these areas. Signal enhancement was also observed in gluteus, vastus lateralis, iliopsoas, and obturator muscles and subcutaneous tissue indicating inflammatory changes.
MRI Coronal T2 Sequence Showing Left Hip Effusion and a Periarticular Collection in Relation With Gluteus Minimus
MRI Axial T2 Sequence Showing Periarticular Abscess and Extensive Affectation of Musculature and Subcutaneous Tissue
MRI Axial T2 Fat Suppression Sequence Showing Edema of Femoral Head and Acetabulum; Extensive Affectation of Periarticular Muscles and Subcutaneous Tissue
MRI Axial T1 Fat Suppression Sequence After IV Gadolinium Showing Peripheral Enhancement of Periarticular Collection and Extensive Osteoarticular and Muscular Signal Changes
The pre-antibiotic blood culture after 17 hours of incubation grew a beta-hemolytic gram positive coccus which was identified by Vitek 2 semi-automatized system as Streptococcus dysgalactiae. Lancefield antigen C was confirmed by a latex agglutination DiaMondial Strep Kit®. The subspecies were identified by means of 16s rRNA sequencing fragment of 1,405 bp using a previously reported method (4). The obtained sequence showed a homology of 98% with SDSE (GenBank accession numbers: AP012976, AP01114, etc.) by the BLAST algorithm. Sensitivity to antibiotic was tested by disc diffusion on Muller Hinton agar; the strain was sensitive to amoxicillin and levofloxacin, but resistant to erythromycin and clindamycin. Culture of surgical synovial fluid samples failed to identify any pathogens.
The antibiotic was then switched to high dose of IV Amoxicillin and all affected teeth were removed. Serum levels of immunoglobulin A, M and G were normal. The little girl progressed well and after 6 weeks of IV antibiotics was discharged on oral Amoxicillin for a further 6 weeks, until the ultrasound examination was normal. She recovered normal function 6 months after onset and no further events have occurred during the one year follow-up.
3. Discussion
The current paper presented the case of a three-year-old toddler with severe osteoarticular and periarticular infection and documented bacteremia by SDSE. This bacteria is increasingly recognized as a dangerous pathogen in humans (5), causing not only severe soft-tissue, musculoskeletal (6, 7) and systemic infections including infant meningitis (8), neonatal streptococcal toxic shock syndrome (STSS) (9), but also post infectious diseases such as rheumatic fever (10), haemolytic uremic syndrome (11) and glomerulonephritis (12). To the authors’ notice SDSE arthritis in children was not reported previously.
SDSE and Streptococcus pyogenes (SP) are evolutionarily related, share the same tissue niche in humans, exchange genetic material and share their virulence-associated genes. In 1996, SDSE was proposed as a novel taxonomic entity among human-derived streptococcal isolates. SDSE has Lancefield group C or G antigens, exhibits strong β-haemolysis, and exerts streptokinase activity on human plasminogen and proteolytic activity on human fibrin. Similar to group A streptococci, SDSE possesses virulence factors including M protein, streptolysin O, streptolysin S, streptokinase, hyaluronidase, C5a peptidase, etc. (13, 14). The current case had an extense bone, joint and soft tissue inflammatory changes suggesting a highly virulent germ as the causing agent.
The niche of SDSE in the patient was assumed to be oral mucosae, presenting multiple teeth decays and history of periodontal phlegons. SDSE is a common colonizer of the oral and pharyngeal mucosa in children (15). It is classically considered a commensal of mucosae and the burden of disease is under recognized (16). Transmission from sites of colonization and infections are the main transmission mechanisms, zoonosis is also being described (17, 18).
SDSE bacteremia is uncommon in children (19); among adults almost all patients have an underlying disease. Predisposing factors for bacteremia and invasive infection are identified, skin breakdown being the most common of them; age over 65 years, diabetes, cardiovascular disease and malignancy or immunosuppression are also described as predisposing factors. The patient had a low socio-economic status but no other predisposing factors were identified, apart from the periodontal disease as a possible cause of bacteremia.
The current case was initially treated on an empiric basis, after isolation of SDSE monotherapy with amoxicillin was indicated. SDSE strains are sensitive to penicillin and beta lactam agents; resistance to macrolides is relatively common (up to 28%), resistance to clindamycin and fluoroquinolones is also described (20). Clindamycin and aminoglycosides could be used in addition to high dose of penicillin in patients with necrotizing fasciitis, streptococcal toxic-shock syndrome (STSS) or severe cellulitis and life threatening infections such as endocarditis or meningitis, respectively (21, 22).
The clinical course was favourable after 12 weeks of antibiotic therapy, until normalization of ultrasound examination. Two month after that, the patient recovered completely and had normal functions. Early surgical intervention and dental extractions during IV antibiotic therapy may have contributed to the good functional outcome.
3.1. Conclusion
Invasive infections due to SDSE are extremely rare in paediatric population. The reported increase in the virulence of this pathogen may lead to more frequent and severe infections in children, known carriers of the bacteria. This is, to the authors’ best knowledge, the first case of septic osteomyelitis and arthritis caused by SDSE described in paediatric population and shows well the potential aggressive course of invasive infection by this emerging pathogen.
Acknowledgements
References
-
1.
Lancefield RC. A Serological Differentiation of Human and Other Groups of Hemolytic Streptococci. J Exp Med. 1933;57(4):571-95. [PubMed ID: 19870148].
-
2.
Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15(4):613-30. [PubMed ID: 12364372].
-
3.
Rantala S. Streptococcus dysgalactiae subsp. equisimilis bacteremia: an emerging infection. Eur J Clin Microbiol Infect Dis. 2014;33(8):1303-10. [PubMed ID: 24682845]. https://doi.org/10.1007/s10096-014-2092-0.
-
4.
Drancourt M, Bollet C, Carlioz A, Martelin R, Gayral JP, Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol. 2000;38(10):3623-30. [PubMed ID: 11015374].
-
5.
Brandt CM, Spellerberg B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin Infect Dis. 2009;49(5):766-72. [PubMed ID: 19635028]. https://doi.org/10.1086/605085.
-
6.
Kumar A, Sandoe J, Kumar N. Three cases of vertebral osteomyelitis caused by Streptococcus dysgalactiae subsp. equisimilis. J Med Microbiol. 2005;54(Pt 11):1103-5. [PubMed ID: 16192443]. https://doi.org/10.1099/jmm.0.46061-0.
-
7.
Raz M, Bilsel K, Ceylan H, Akkoyunlu Y. Streptococcus dysgalactiae Subspecies Equisimilis; An Agent Rarely Encountered in the Etiology of Septic Arthritis. Jundishapur J Microbiol. 2013;6(7). eee9175.
-
8.
Quinn RJ, Hallett AF, Appelbaum PC, Cooper RC. Meningitis caused by Streptococcus dysgalactiae in a preterm infant. Am J Clin Pathol. 1978;70(6):948-50. [PubMed ID: 569439].
-
9.
Yamaoka S, Ogihara T, Yasui M, Hasegawa M, Hira S, Oue S, et al. Neonatal streptococcal toxic shock syndrome caused by Streptococcus dysgalactiae subsp. equisimilis. Pediatr Infect Dis J. 2010;29(10):979-81. [PubMed ID: 20879096].
-
10.
Haidan A, Talay SR, Rohde M, Sriprakash KS, Currie BJ, Chhatwal GS. Pharyngeal carriage of group C and group G streptococci and acute rheumatic fever in an Aboriginal population. Lancet. 2000;356(9236):1167-9. https://doi.org/10.1016/s0140-6736(00)02765-3.
-
11.
Galan-Sanchez F, Guerrero-Lozano I, Rubio-Quinones F, Rodriguez-Iglesias M. Haemolytic uraemic syndrome associated with bloody diarrhoea caused by Streptococcus dysgalactiae. Enferm Infecc Microbiol Clin. 2013;31(1):60-1. [PubMed ID: 22955002]. https://doi.org/10.1016/j.eimc.2012.07.001.
-
12.
Reid HFM, Bassett DCJ, Poon-King T, Zabriskie JB, Read SE. Group G streptococci in healthy school-children and in patients with glomerulonephritis in Trinidad. Journal of Hygiene. 2009;94(1):61. https://doi.org/10.1017/s0022172400061131.
-
13.
Takahashi T, Ubukata K, Watanabe H. Invasive infection caused by Streptococcus dysgalactiae subsp. equisimilis: characteristics of strains and clinical features. J Infect Chemother. 2011;17(1):1-10. [PubMed ID: 20607346]. https://doi.org/10.1007/s10156-010-0084-2.
-
14.
Gherardi G, Imperi M, Palmieri C, Magi G, Facinelli B, Baldassarri L, et al. Genetic diversity and virulence properties of Streptococcus dysgalactiae subsp. equisimilis from different sources. J Med Microbiol. 2014;63(Pt 1):90-8. [PubMed ID: 24149625]. https://doi.org/10.1099/jmm.0.062109-0.
-
15.
Bramhachari PV, Kaul SY, McMillan DJ, Shaila MS, Karmarkar MG, Sriprakash KS. Disease burden due to Streptococcus dysgalactiae subsp. equisimilis (group G and C streptococcus) is higher than that due to Streptococcus pyogenes among Mumbai school children. J Med Microbiol. 2010;59(Pt 2):220-3. [PubMed ID: 19833781]. https://doi.org/10.1099/jmm.0.015644-0.
-
16.
Zaoutis T, Attia M, Gross R, Klein J. The role of group C and group G streptococci in acute pharyngitis in children. Clin Microbiol Infect. 2004;10(1):37-40. [PubMed ID: 14706084].
-
17.
Pinho MD, Matos SC, Pomba C, Lubke-Becker A, Wieler LH, Preziuso S, et al. Multilocus sequence analysis of Streptococcus canis confirms the zoonotic origin of human infections and reveals genetic exchange with Streptococcus dysgalactiae subsp. equisimilis. J Clin Microbiol. 2013;51(4):1099-109. [PubMed ID: 23345291]. https://doi.org/10.1128/JCM.02912-12.
-
18.
Schrieber L, Towers R, Muscatello G, Speare R. Transmission of Streptococcus dysgalactiae subsp. equisimilis between child and dog in an Aboriginal Australian community. Zoonoses Public Health. 2014;61(2):145-8. [PubMed ID: 23745768]. https://doi.org/10.1111/zph.12057.
-
19.
Ekelund K, Skinhoj P, Madsen J, Konradsen HB. Invasive group A, B, C and G streptococcal infections in Denmark 1999-2002: epidemiological and clinical aspects. Clin Microbiol Infect. 2005;11(7):569-76. [PubMed ID: 15966976]. https://doi.org/10.1111/j.1469-0691.2005.01169.x.
-
20.
Broyles LN, Van Beneden C, Beall B, Facklam R, Shewmaker PL, Malpiedi P, et al. Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis. 2009;48(6):706-12. [PubMed ID: 19187026]. https://doi.org/10.1086/597035.
-
21.
Coyle EA, Cha R, Rybak MJ. Influences of linezolid, penicillin, and clindamycin, alone and in combination, on streptococcal pyrogenic exotoxin a release. Antimicrob Agents Chemother. 2003;47(5):1752-5. [PubMed ID: 12709354].
-
22.
Lam K, Bayer AS. In vitro bactericidal synergy of gentamicin combined with penicillin G, vancomycin, or cefotaxime against group G streptococci. Antimicrob Agents Chemother. 1984;26(2):260-2. [PubMed ID: 6091537].