Abstract
Background:
Primary immunodeficiency disorders comprise a heterogeneous group of diseases in which there is a defect in the development or function of the immune system. Clinical presentation of these disorders is highly variable. The aim of this study was to determine the frequency and type of primary immunodeficiency disorders, diagnostic delay, common pathogenic microorganisms, and infectious complications in patients, admitted to Mofid Children’s hospital in the past decade (2004 - 2014).Methods:
The data of patients with a diagnosis of primary immunodeficiency disorder, admitted to Mofid Children’s hospital from 2004 to 2014, were reviewed. The frequency, diagnostic delay, pathogenic microorganisms, and their sequelae were determined.Results:
In 32 patients (20 males and 12 females) admitted to Mofid hospital in the past decade, chronic granulomatous disease was the most frequent primary immunodeficiency disorder (22%). Other immunodeficiency disorders in these patients included X-linked agammaglobulinemia, hyper IgM syndrome, common variable immunodeficiency, severe combined immunodeficiency, ataxia-telangiectasia syndrome, transient hypogammaglobulinemia of infancy, leukocyte adhesion deficiency, and Kostmann syndrome. The mean age at onset of these disorders was 15 months, and the mean delay in diagnosis was 20 months. Pneumonia was the most common infectious manifestation (53%), and Acinetobacter was the most common isolated microorganism.Conclusions:
Diagnostic delay is a major concern in patients with primary immunodeficiency disorders. Ethnic and geographical differences may significantly influence the frequency and presentation of these disorders. Therefore, local epidemiological data are always necessary and useful in the management of primary immunodeficiency disorders.Keywords
Immunologic Deficiency Syndromes PID Delayed Diagnosis Iran Epidemiology
1. Background
Primary immunodeficiency (PID) diseases comprise a heterogeneous group of disorders. Clearly, these diseases are not as rare as originally suspected. The spectrum of PID disorders has greatly widened in the past few decades, with more than 200 different disorders or syndromes, affecting the immune system development, function, or morphology (1, 2). It is well accepted that clinical presentations of PID disorders are highly variable and include inflammatory, autoimmune, allergic, and lymphoproliferative symptoms, as well as susceptibility to recurrent infections, which is the hallmark of these disorders (3, 4). Diagnosis of PID disorders is established only if the physician is alert to that possibility.
PID disorders are classified into 8 main categories: 1) combined T- and B-cell immunodeficiencies; 2) antibody deficiencies; 3) well-defined immunodeficiency syndromes; 4) diseases of immune dysregulation; 5) defects of phagocyte number, function, or both; 6) defects in innate immunity; 7) autoinflammatory disorders; and 8) complement deficiencies (5).
Delay in the diagnosis of PID disorders is not only evident in undeveloped or developing countries, but is also reported in developed countries. On the other hand, early diagnosis and easy access to care and treatment play key roles in improving survival, quality of life, and prognosis of these disorders (6). A national survey of PID disorders in the United States published in 2011 (7) found that more than 40% of patients with these disorders were not diagnosed until adulthood, despite frequent reports of serious or chronic health conditions, such as sinusitis, bronchitis, and pneumonia prior to diagnosis (7).
Delay in diagnosis and inadequate management may lead to permanent organ damage, such as bronchiectasis and bronchiolitis obliterans, or even death from overwhelming sepsis (1). With this background in mind, we designed this study to evaluate the features of PID disorders and duration of diagnostic delay in patients admitted to Mofid Children’s hospital.
2. Methods
Among patients referred to Mofid Children’s hospital during 10 years, 32 patients had a diagnosis of PID disorders. These patients were suspected to have PID disorders according to clinical findings, such as recurrent episodes of infection and infections with low virulence microorganisms, unusual microorganisms, unusual severity, and atypical sites.
The subjects underwent further evaluation and immunodeficiency screening tests, including complete blood count (CBC), serum immunoglobulin level, antibody response to immunization, serum complement concentration, nitroblue tetrazolium test, and flow cytometric evaluation. Mutation analysis was also performed to confirm the diagnosis. A questionnaire with detailed information regarding gender, age, and age of onset of primary PID manifestations was completed for each patient. Since data collection included clinical records, gathered by a trained resident of pediatrics, with no additional interventions or even mentioning the patients' names, the ethics committee of Mofid Teaching Hospital did not consider formal patient consents necessary.
2.1. Statistical Analysis
Data analysis was performed using SPSS version 16. Comparisons between quantitative data, such as age at onset of infection, age at diagnosis, and delay in diagnosis, were performed by measuring the mean, mode, median, and standard deviation. Nominal qualitative data, including sex, site of infection, and pathogenic microorganisms isolated from the patients, were analyzed. The collected data are presented as tables and diagrams.
3. Results
In this study, 32 patients with PID disorders, admitted to Mofid Children’s hospital, were evaluated. In total, 20 (62%) patients were male and the remaining (n, 12; 38%) were female. The male-to-female ratio was calculated to be 1:66. Among all the evaluated patients, the mean age at onset of PID disorders was 15.03 months with a standard deviation of 18.51. Diagnosis delay refers to the interval between the first episode of infection and diagnosis. The mean delay in the diagnosis of PID disorders was 20.1 months with a standard deviation of 18.29.
The clinical manifestations initiated before 10 months of age in 56% of patients. In 22% of patients, clinical presentations were first observed at 10 to 20 months. Diagnosis in 50%, 22%, and 19% of patients was established before 25, 25 - 50, and 50 - 75 months of age, respectively. Diagnostic delay was less than 10 months in 47% of patients and 10 - 60 months in the remaining patients (53%). The time interval between the primary manifestations and definite diagnosis was 41 months for X-linked agammaglobulinemia, 62 months for common variable immunodeficiency, and 79 months for chronic granulomatous disease. Moreover, 15% of these patients died because of the associated complications.
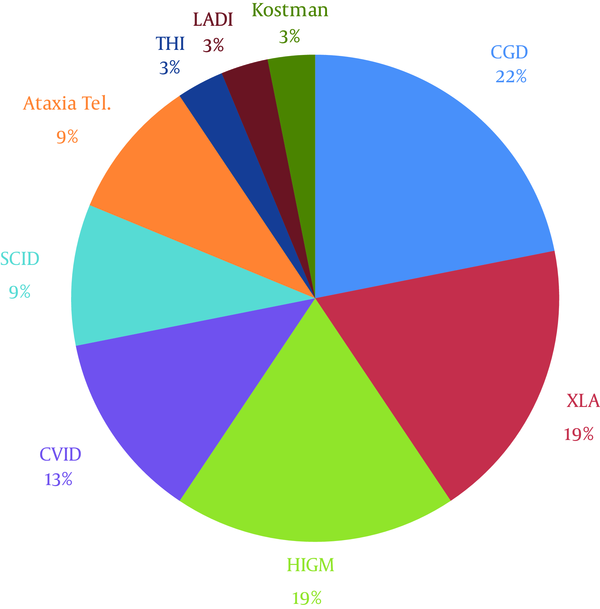
The distribution of PID diagnoses in this study is demonstrated in Figure 1. The most common infectious complications were pneumonia (53.12%), gastroenteritis (40.62%), otitis (21.87%), mouth ulcer (21.87%), sinusitis (18.75%), cervical lymphadenitis and mastoiditis (15.62% each), sepsis (12.5%), septic arthritis (12.5%), inguinal and perineal abscesses (12.5%), disseminated bacillus Calmette-Guérin (BCG) infection (9.37%), common cold and influenza infection (9.37%), lung abscess, candidiasis, and cryptosporidium (6.25% each).
Distribution of PID Diagnoses in Mofid Children’s Hospital During 2004 - 2014
The most frequently isolated microorganisms were Acinetobacter (18.75%), Staphylococcus aureus and Cryptosporidium (15.6% each), Pseudomonas and Giardia (9.4% each), Aspergillosis, Enterobacter, Enterococcus, Candida, Mycobacterium tuberculosis, and Staphylococcus epidermidis (6.25% each), Cytomegalovirus (3.12%), Streptococcus group C and G, Mucormycosis, Staphylococcus auricularis, Klebsiella, Mycoplasma, and Streptococcus pneumoniae (3.12% each). Common infections in specific PID disorders and the isolated microorganisms are presented in Tables 1 and 2.
The Frequency of Infectious Episodes in PID Disorders
| PID Disorders | Type of Infections (Number of Patients) |
|---|---|
| Chronic granulomatous disease | Pneumonia (3), lymphadenitis (3), inguinal and perianal abscesses (2), lung abscess (2), BCG-osis (2), peritonitis (1), mouth ulcers (1), sinusitis (1), mastoiditis (1), and respiratory infections (1) |
| X-linked agammaglobulinemia | Pneumonia (5), gastroenteritis (4), otitis (4), septic arthritis (3), respiratory infections (2), sinusitis (1), mastoiditis (1), and sepsis (1) |
| Hyper IgM syndrome | Pneumonia (4), gastroenteritis (3), sinusitis (3), mouth ulcers (3), cryptosporidial gastroenteritis (1), common cold (1), colitis (1), otitis (1), and septic arthritis (1) |
| Common variable immunodeficiency | Gastroenteritis (4), pneumonia (2), sinusitis (2), cryptosporidial gastroenteritis (1), mastoiditis (1), mouth ulcer (1), lymphadenitis (1), meningitis (1), and cytomegalovirus gastroenteritis (1) |
| Severe combined immunodeficiency | Gastroenteritis (2), pneumonia (2), sepsis (2), mouth ulcer (1), lymphadenitis (1), and BCG-osis (1) |
| Ataxia-telangiectasia syndrome | Respiratory infections (3), pneumonia (2), gastroenteritis (1), and mouth ulcer (1) |
| Transient hypogammaglobulinemia of infancy | Inguinal and perianal abscesses (1) |
| BCG-osis | Pneumonia (1), inguinal and perianal abscesses (1), respiratory infections (1), and lung abscess (1) |
| Leukocyte adhesion deficiency | Inguinal and perianal abscesses (1), otitis (1), and mastoiditis (1) |
| Kostmann syndrome | Sepsis (1), otitis (1), and mastoiditis (1) |
The Frequency of Microorganisms in PID Disorders
| PID Diseases | Pathogenic Microorganisms (Number of Patients) |
|---|---|
| Chronic granulomatous disease | Staphylococcus aureus (3), M. tuberculosis (2), Aspergillus (1), Enterobacter (1), and Enterococcus (1) |
| X-linked agammaglobulinemia | Giardia (3), Acinetobacter (2), Staphylococcus aureus (2), Pseudomonas (1), Staphylococcus epidermidis (1), and Klebsiella (1) |
| Hyper IgM syndrome | Cryptosporidium (3), Acinetobacter (2), and Candida (1) |
| Common variable immunodeficiency | Cryptosporidium (3), Shigella (1), and cytomegalovirus (1) |
| Severe combined immunodeficiency | Pseudomonas (2), Enterobacter (1), Acinetobacter (1), respiratory syncytial virus (1), and Streptococcus group C (1) |
| Ataxia-telangiectasia syndrome | Mycoplasma (1), Streptococcus pneumoniae (1), and Candida (1) |
| Transient hypogammaglobulinemia of infancy | Staphylococcus epidermidis (1) |
| BCG-osis | M. tuberculosis (1) |
| Leukocyte adhesion deficiency | Staphylococcus auricularis (1) and Enterococcus (1) |
| Kostmann syndrome | Pseudomonas (1), Aspergillosis (1), and mucormycosis (1) |
4. Discussion
Although PID disorders are rare, diverse genetic defects may result in a wide range of clinical symptoms. Due to lack of knowledge and unawareness of medical personnel about PID disorders, diagnosis is often made with some delays.
In a study performed at different universities of medical sciences in 5 different regions of Iran in 2002 (8), data of patients with documented PID disorders according to the WHO criteria (9) were collected in a database center with an access database software under the name, “Iranian primary immunodeficiency registry”. The information of 440 patients with PID disorders during the past 2 decades was recorded in this center. PID disorders in these patients included antibody deficiency (46%), phagocytic defects (29%), T-cell deficiencies (24.5%), and complement deficiencies (0.5%), respectively. The majority of these patients were admitted to children’s hospitals, and more than 63% were in the pediatric age group. The male-to-female ratio was 1.7:1.
In another study performed by the same group of investigators in Iran in 2011 on 930 PID patients (5), delay in diagnosis was significantly reduced. Delay in diagnosis was 7 years, 3 years, and 15 months before 1990, during 1990 - 1995, and after 1995, respectively, which demonstrates a significant reduction in diagnostic delay in recent decades perhaps due to the availability of advanced laboratory investigations. Mortality of patients was estimated at 17.2% in this study (5). An interesting finding observed among 515 patients over the past 25 years was parental consanguinity in 65.6% of cases, while the rate of consanguinity in the general population was estimated at 38.6% in Iran.
In a clinical survey by Moin et al. on Iranian patients with hyper IgE syndrome, a median diagnostic delay of 70 months was reported (10). In order to determine the incidence of PID disorders and examine the association between delayed diagnosis and increased morbidity, a cohort study was performed during 31 years in Olmsted County, Minnesota, USA from 1976 to 2006. The results indicated that a longer delay in diagnosis was significantly associated with recurrent sinusitis and recurrent pneumonia. Moreover, older age at diagnosis was significantly associated with mortality (11).
In a study performed in 2011 in Iran (11), Aghamohammadi et al. demonstrated a close inverse correlation between the frequency of infection and quality of life. A similar association was also documented between delay in diagnosis of PID disorders and quality of life among patients. They concluded that earlier diagnosis was associated with a majorly higher quality of life. On the other hand, patients with a more severe clinical phenotype and protracted diagnostic delay had a majorly lower quality of life (12).
In another study performed in Iran, the mean diagnostic delay in 48 patients with primary antibody deficiency was more than 45 months (5). Physicians’ awareness of PID disorders is important in timely diagnosis and prevention of diagnostic delay and its consequences in these patients. Despite the general availability of immunological diagnostic techniques, PID disorders are still diagnosed with delay; in many patients, they are diagnosed after irreversible structural organ damage.
Additionally, Nourijelyani et al. (12) designed a study with a questionnaire consisting of 52 questions on clinical manifestations, laboratory data, relevant syndromes, and management of PID disorders. A total of 333 physicians participated in this study, while only 32% of physicians correctly answered more than two-thirds of the questions. This study highlighted the unawareness of general physicians about PID disorders. This lack of knowledge can negatively influence diagnostic delay and appropriate management of these patients (13).
In the present study, the male-to-female ratio was 1:66. Primary studies in this area reported male-to-female ratios of 2:1 to 1.4:1 (10, 11). This finding is compatible with the data reported by an Iranian registry in 2006 (5), which demonstrated that more than half of these patients are still males, despite the global increase in identification of female patients with PID disorders.
The mean diagnostic delay from the first presentation of disease until final diagnosis was 20 months in our study. This is indicative of a decreased diagnostic delay, compared with previous studies. The decreased duration of diagnostic delay might be attributed to parameters, such as an increased index of suspicion in primary care physicians, establishment of referral centers for the management of suspected patients, and better access to different treatment modalities for these patients, compared to the past.
Since the onset of symptoms is generally between 15 and 40 years in common variable immunodeficiency, a diagnostic delay of 5 - 8 years after the first cardinal symptom is common in these patients in all countries. This could be another explanation for the decreased diagnostic delay in our patients, which might be influenced by the fewer number of patients with common variable immunodeficiency in the present study.
It should be noted that the available data do not provide an actual presentation of the prevalence of PID disorders in Iran, since the registered data are based on reports and review of information from hospitalized patients. At present, the data of deceased patients are not recorded, which can result in an underestimation of the actual prevalence of PID disorders; therefore, advanced registry systems and newborn screening programs need to be implemented.
Furthermore, early and proper management of patients with PID disorders remains the main problem of these patients in Iran. This can be partly explained by the unawareness of general practitioners, difficulties in access to screening tests, and lack of specialized treatment centers in many regions of the country; therefore, many patients remain undiagnosed. In addition, it seems that patients with severe manifestations in remote parts of the country succumb to these disorders in infancy without a proper diagnosis.
Although diagnostic delay in PID disorders has decreased significantly, the occurrence rate remains high. Physicians' awareness of the prevalence of PID disorders, common associated infections, and pathogenic microorganisms may result in earlier diagnosis and application of appropriate treatments before end organ damage.
Acknowledgements
References
-
1.
Edgar JD, Buckland M, Guzman D, Conlon NP, Knerr V, Bangs C, et al. The United Kingdom Primary Immune Deficiency (UKPID) Registry: report of the first 4 years' activity 2008-2012. Clin Exp Immunol. 2014;175(1):68-78. [PubMed ID: 23841717]. https://doi.org/10.1111/cei.12172.
-
2.
Jesenak M, Banovcin P, Jesenakova B, Babusikova E. Pulmonary manifestations of primary immunodeficiency disorders in children. Front Pediatr. 2014;2:77. [PubMed ID: 25121077]. https://doi.org/10.3389/fped.2014.00077.
-
3.
Errante PR, Franco JL, Espinosa-Rosales FJ, Sorensen R, Condino-Neto A. Advances in primary immunodeficiency diseases in Latin America: epidemiology, research, and perspectives. Ann N Y Acad Sci. 2012;1250:62-72. [PubMed ID: 22364447]. https://doi.org/10.1111/j.1749-6632.2011.06289.x.
-
4.
Parvaneh N, Casanova JL, Notarangelo LD, Conley ME. Primary immunodeficiencies: a rapidly evolving story. J Allergy Clin Immunol. 2013;131(2):314-23. [PubMed ID: 23374262]. https://doi.org/10.1016/j.jaci.2012.11.051.
-
5.
Aghamohammadi A, Bahrami A, Mamishi S, Mohammadi B, Abolhassani H, Parvaneh N, et al. Impact of delayed diagnosis in children with primary antibody deficiencies. J Microbiol Immunol Infect. 2011;44(3):229-34. [PubMed ID: 21524619]. https://doi.org/10.1016/j.jmii.2011.01.026.
-
6.
Buldeo S, Murdoch DM, Suchard MS. Pulmonary immune-compartment-specific interferon gamma responses in HIV-infected individuals with active tuberculosis (TB) in an area of high TB prevalence. Clin Dev Immunol. 2012;2012:308473. [PubMed ID: 22778764]. https://doi.org/10.1155/2012/308473.
-
7.
McCusker C, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol. 2011;7 Suppl 1. S11. [PubMed ID: 22165913]. https://doi.org/10.1186/1710-1492-7-S1-S11.
-
8.
Aghamohammadi A, Moein M, Farhoudi A, Pourpak Z, Rezaei N, Abolmaali K, et al. Primary immunodeficiency in Iran: first report of the National Registry of PID in Children and Adults. J Clin Immunol. 2002;22(6):375-80. [PubMed ID: 12462337].
-
9.
Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, Ames BN. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res. 1996;351(2):199-203. [PubMed ID: 8622715].
-
10.
Moin M, Farhoudi A, Movahedi M, Rezaei N, Pourpak Z, Yeganeh M, et al. The clinical and laboratory survey of Iranian patients with hyper-IgE syndrome. Scand J Infect Dis. 2006;38(10):898-903. [PubMed ID: 17008235]. https://doi.org/10.1080/00365540600740470.
-
11.
Joshi AY, Iyer VN, Hagan JB, St Sauver JL, Boyce TG. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clin Proc. 2009;84(1):16-22. [PubMed ID: 19121249]. https://doi.org/10.1016/S0025-6196(11)60802-1.
-
12.
Aghamohammadi A, Montazeri A, Abolhassani H, Saroukhani S, Pourjabbar S, Tavassoli M, et al. Health-related quality of life in primary antibody deficiency. Iran J Allergy Asthma Immunol. 2011;10(1):47-51. [PubMed ID: 21358015].
-
13.
Nourijelyani K, Aghamohammadi A, Salehi Sadaghiani M, Behniafard N, Abolhassani H, Pourjabar S, et al. Physicians awareness on primary immunodeficiency disorders in Iran. Iran J Allergy Asthma Immunol. 2012;11(1):57-64. [PubMed ID: 22427477].




