Abstract
Keywords
1. Introduction
Brucellosis is the most prevalent zoonotic infection in Iran, especially in west and northwest regions of the country (1). The high prevalence of brucellosis has made this infection a public health concern (2). In children, it is associated with fever, sweating, hepatosplenomegaly, and arthritis of large joints. Anemia, leukopenia or leukocytosis, thrombocytopenia, and increased concentration of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and liver function tests are the most common laboratory findings on brucellosis assessment (3). Hemophagocytic lymphohistiocytosis (HLH) represents non-malignant generalized proliferation of histiocytes, with marked hemophagocytosis in the liver, bone marrow, spleen, and central nervous system (4-6).
As mentioned earlier, cytopenia, high fever, and hepatosplenomegaly are common presentations of brucellosis, while pancytopenia is not common in this infection (7, 8). Herein, we present a case of secondary HLH associated with brucellosis.
2. Case Presentation
A three-year-old boy was admitted to our hospital with one month of fever, cough, and fatigue, without weight loss or arthralgia. He was hospitalized for pervious 2 weeks in another hospital for evaluation of FUO and cytopenia. The results of pervious evaluation showed pancytopenia, fasting triglyceride (TG) level: 338 mg/dL, ferritin level of > 800 ng/mL, and bone marrow aspiration (BMA) reported normal.
In his past medical history, consumption of ice cream, as an unpasteurized milk product, was reported. Based on examination upon admission, he was conscious, well-oriented, pale, and febrile (about 39°C), with a pulse rate of 120/min, blood pressure of 96/65 mmHg, respiratory rate of 26/min, and capillary refill time of < 3 seconds. Spleen and liver palpated 4 cm and 11 cm below the costal margin respectively. We repeated the evaluations by ordering complete blood cell count (CBC), Blood culture, aspartate transaminase (AST) and alanine transaminase (ALT) levels, peripheral blood smear for Malaria, Tuberculin skin test (TST), Wright and Coombs Wright, viral capsid antibody (VCA), and anti cytomegalovirus antibodies, ESR and CRP.
According to our analyses, the level of hemoglobin was 8.1 mg/mL the total leukocyte count (WBC) was 4100/mm3 (neutrophil = 27%; lymphocyte = 70%), and the platelet count was 38000/mm3. ESR and CRP were 37 mm/hour and 53 mg/dL, respectively. The biochemical analysis showed (AST) (ALT) levels of 330 U/L and 74 U/L, respectively. Based on our findings, the Wright and Coombs Wright test titers were 1:640 and 1:320, respectively, and the 2-mercaptoethanol (2ME) test result was 1:160. The rest of results were normal.
Treatment with rifampin and trimethoprim-sulfamethoxazole was initiated. During the first 5 days of treatment the child became more ill and could not eat and play like the admission time and the high-grade fevers (more than 39.5) were reported every four hours. So, this child was evaluated for differential diagnosis (like malignancy, rheumatologic disorders, human immunodeficiency virus, and hemophagocytic lymphohistiocytosis). Considering the patient’s unresponsiveness to treatment and the gradual increase brucella resistance to cotrimoxazole and rifampin in Iran (9), these drugs were discontinued for the patient, and doxycycline, ciprofloxacin, and gentamicin were initiated. In 10th day of treatment the clinical course did not change and spleen palpated 6 cm below the costal margin. The lab data results reported: Bactec was negative, 2100/mm3 (neutrophil = 27%; lymphocyte = 70%), platelet count: 30000/mm3. serum fasting triglyceride level of 444 mg/dL, a ferritin level of > 2800 ng/mL, and a fibrinogen level of 105 mg/dL. ESR: 30 mm/hour and CRP: 50 mm/hour. In addition, HBsAg and HIV antibodies were negative.
Antinuclear antibody (ANA), serum hemolytic complement (CH50), anti-dsDNA, C3, and C4 levels were in the normal range. Malaria with Borrelia species was negative in the peripheral blood smear.
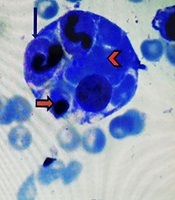
In accordance to deuteriation of patient, increase spleen size, increase in ferritin level, decrease in ESR level, pancytopenia and prolonged course of brucellosis, infectious associated HLH was one the diagnosis (8). According to pediatric hematologist BMA again was done and intravenous immunoglobulin (IVIG) and dexamethasone were initiated. IVIG was administered at a dose of 400 mg/kg for four consecutive days, in addition to antimicrobial therapy for secondary HLH. After two days, fever disappeared, and his clinical symptoms improved. Hemophagocytosis was observed by pathologist and the diagnosis of secondary HLH confirmed (Figure 1).
A Hemophagocytic lymphohistiocytosis cell

During 14 days of hospitalization with continuation of treatment, the patient completely recovered. The size of the liver and the spleen decreased, and laboratory findings changed. Moreover, oral doxycycline (for one week), ciprofloxacin and rifampin (for 4 weeks) were administered for treatment, and a tapering dose of dexamethasone was initiated according to the treatment protocols (5). The patient was recommended an outpatient follow-up visit after two weeks.
Now after one year fallow up the child is well without any problem, and the immune system evaluation results reported normal.
3. Discussion
Neurological damage, musculoskeletal involvement, and cytopenia due to bone marrow involvement are complications of brucellosis, with a prevalence rate of approximately 5% - 10% (6). Pancytopenia or platelet count reduction rarely occurs in brucellosis. Pancytopenia has multiple pathogeneses, including hemophagocytosis, hypersplenism, bone marrow deterioration, and immune system insufficiency (7, 10). Few studies have reported an association between this multi-organ infection and cytopenia, pancytopenia, or hemophagocytosis (6, 7, 11, 12). Fever, pancytopenia, and splenomegaly, as common signs and symptoms of brucellosis, are also among the main diagnostic criteria for HLH; therefore, diagnosis of this infection can be challenging for physicians.
In this regard, Karakulukcu et al. reported four pediatric patients with Brucella-associated HLH. The patients presented with pancytopenia and received specific antimicrobial agents for Brucella infection; they fully recovered after appropriate treatment for the infection. In our patient, not only anti-brucellosis therapy was initiated immediately upon admission, but also it was modified due to the possibility of drug resistance (9). However, antimicrobial treatment failed, and the patient’s condition deteriorated; since the patient met the diagnostic criteria for HLH, dexamethasone and IVIG were initiated.
Some scholars recommend IVIG and dexamethasone for the treatment of secondary HLH associated with Brucella infection (11, 13, 14). The HLH is hyperinflammation due to increase in cytokines levels (13). Immunomodulators for HLH therapy reduce these cytokines strom (13). Generally, duration of secondary pancytopenia in Brucella infection varies from eight days to three weeks after antimicrobial therapy (8, 11). Nevertheless, the time required to achieve responsiveness to IVIG (a recommended medication for HLH following Brucella infection) is nearly three to seven days (11, 13, 14). It is worth mentioning that this period was exactly the time needed to achieve recovery in our patient, who was treated with these medications. Therefore, use of IVIG seems to be effective as an emergency treatment for life-threatening conditions in patients with pancytopenia or thrombocytopenia, secondary to brucellosis or Brucella-related HLH (11-17). Also, corticosteroids or immunosuppressive drugs should be used as therapeutic agents for overproduction of cytokines due to excessive inflammation in HLH (4). It should be noted that treating the underlying infection, associated with secondary HLH, can be useful in combination with other medications (18).
3.1. Conclusions
Secondary HLH should be considered in patients with brucellosis, who present with prolonged fever, organomegaly, cytopenia, and poor response to standard anti-Brucella treatment.
References
-
1.
Mirnejad R, Jazi FM, Mostafaei S, Sedighi M. Epidemiology of brucellosis in Iran: A comprehensive systematic review and meta-analysis study. Microb Pathog. 2017;109:239-47. [PubMed ID: 28602839]. https://doi.org/10.1016/j.micpath.2017.06.005.
-
2.
Rastegar Lari A, Abdollah Karimi A, Goli Angotti F, Sanaei A, Azimi L. The efficacy of multiplex PCR in comparison with agglutination and ELISA in the diagnosis of human Brucellosis. Iran J Clin Infect Dis. 2011;6((suppl)).
-
3.
Caylak SD. A brucellosis case presenting with vesicular and maculopapular rash and febrile neutropenia. J Microbiol Infect Dis. 2014;4(1):39-41. https://doi.org/10.5799/ahinjs.02.2014.01.0123.
-
4.
Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-31. [PubMed ID: 16937360]. https://doi.org/10.1002/pbc.21039.
-
5.
Larroche C, Mouthon L. Pathogenesis of hemophagocytic syndrome (HPS). Autoimmun Rev. 2004;3(2):69-75. [PubMed ID: 15003190]. https://doi.org/10.1016/S1568-9972(03)00091-0.
-
6.
Akdeniz H, Irmak H, Seckinli T, Buzgan T, Demiröz AP. Hematologic changes in brucellosis cases in Turkey. Acta Med Okayama. 1998;52:63-5.
-
7.
Akbayram S, Dogan M, Akgun C, Peker E, Parlak M, Caksen H, et al. An analysis of children with brucellosis associated with pancytopenia. Pediatr Hematol Oncol. 2011;28(3):203-8. [PubMed ID: 21381870]. https://doi.org/10.3109/08880018.2010.536298.
-
8.
Erduran E, Makuloglu M, Mutlu M. A rare hematological manifestation of brucellosis: Reactive hemophagocytic syndrome. J Microbiol Immunol Infect. 2010;43(2):159-62. [PubMed ID: 20457434]. https://doi.org/10.1016/S1684-1182(10)60025-4.
-
9.
Torkaman Asadi F, Hashemi SH, Alikhani MY, Moghimbeigi A, Naseri Z. Clinical and diagnostic aspects of brucellosis and antimicrobial susceptibility of Brucella isolates in Hamedan, Iran. Jpn J Infect Dis. 2017;70(3):235-8. [PubMed ID: 27580575]. https://doi.org/10.7883/yoken.JJID.2016.133.
-
10.
Karakukcu M, Patiroglu T, Ozdemir MA, Gunes T, Gumus H, Karakukcu C. Pancytopenia, a rare hematologic manifestation of brucellosis in children. J Pediatr Hematol Oncol. 2004;26(12):803-6. [PubMed ID: 15591899].
-
11.
Citak EC, Citak FE, Tanyeri B, Arman D. Hematologic manifestations of brucellosis in children: 5 years experience of an anatolian center. J Pediatr Hematol Oncol. 2010;32(2):137-40. [PubMed ID: 20147850]. https://doi.org/10.1097/MPH.0b013e3181ced382.
-
12.
David A, Iaria C, Giordano S, Iaria M, Cascio A. Secondary hemophagocytic lymphohistiocytosis: Forget me not!. Transpl Infect Dis. 2012;14(5):E121-3. [PubMed ID: 22852815]. https://doi.org/10.1111/j.1399-3062.2012.00770.x.
-
13.
Yaman Y, Gozmen S, Ozkaya AK, Oymak Y, Apa H, Vergin C, et al. Secondary hemophagocytic lymphohistiocytosis in children with brucellosis: Report of three cases. J Infect Dev Ctries. 2015;9(10):1172-6. [PubMed ID: 26517495]. https://doi.org/10.3855/jidc.6090.
-
14.
El Euch M, Kaabar MY, Bouaziz R, Mahfoudhi M, Jaziri F, Kefi A, et al. Successful resolution of Hemophagocytic lymphohistiocytosis associated to brucellosis in the adult. Tunis Med. 2018;96(7):458-61. [PubMed ID: 30430494].
-
15.
Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S82-9. [PubMed ID: 19932759]. https://doi.org/10.1016/j.bbmt.2009.11.014.
-
16.
Nomura S, Koshikawa K, Hamamoto K, Okubo S, Yasunaga K. [Steroid and gamma globulin therapy against virus-associated hemophagocytic syndrome]. Rinsho Ketsueki. 1992;33(9):1242-7. Japanese. [PubMed ID: 1279236].
-
17.
Chen RL, Lin KH, Lin DT, Su IJ, Huang LM, Lee PI, et al. Immunomodulation treatment for childhood virus-associated haemophagocytic lymphohistiocytosis. Br J Haematol. 1995;89(2):282-90. [PubMed ID: 7873378]. https://doi.org/10.1111/j.1365-2141.1995.tb03302.x.
-
18.
Imashuku S, Teramura T, Morimoto A, Hibi S. Recent developments in the management of haemophagocytic lymphohistiocytosis. Expert Opin Pharmacother. 2001;2(9):1437-48. [PubMed ID: 11585022]. https://doi.org/10.1517/14656566.2.9.1437.