Abstract
Background:
At present, some evidence supports the role of Helicobacter pylori eradication in treating childhood immune thrombocytopenia (ITP).Objectives:
This study was designed to investigate the association between H. pylori eradication and platelet count in children with acute ITP.Methods:
Thirty children with a diagnosis of acute ITP and H. pylori infection were studied. This randomized and controlled study was done in Amir-Kabir Hospital, Arak, Iran. Patients were randomly allocated 1:1 to standard ITP and H. pylori treatments or standard ITP treatment. For 6 months, studied subjects had monthly follow-ups.Results:
The mean ± standard deviation (SD) age was 11 years with a majority of females (67.9 % vs. 32.1 %). The mean platelet count was 16.93 ± 10.1 (109/L) in the ITP and H. pylori therapy group, and15.0 ± 3.8 (109/L) in the ITP therapy group at the baseline. After 1 week of treatment, mean platelet counts increased to 136.5 ± 55.20 and 124.0 ± 45.70 in the two groups, respectively (109/L). The differences between the two groups were not statistically significant at other time intervals. Children that received ITP and H. pylori therapies had a higher rate of gastrointestinal symptoms (P < 0.01).Conclusions:
Our findings suggest that H. pylori eradication did not significantly affect the platelet count compared to standard ITP therapy in children with acute ITP.Keywords
1. Background
Immune thrombocytopenia (ITP) is an immune disorder in adults and children, causing a decrease in the number of platelets (1). Autoantibodies are responsible for platelet coating, which are readily removed by the liver and spleen (1, 2). The ITP is defined as the platelet count less than 100 × 109/L with normal white blood cell number and normal hemoglobin in the absence of other disorders, causing thrombocytopenia. The ITP can be acute, which is usually associated with infection and can be chronic, which can be continued for more than 1 year (3, 4). Glucocorticoids, anti-D globulin, and IV immune globulin are therapeutic choices for ITP (5). Helicobacter pylori (H. pylori) is a gram-negative bacillus that colonizes the gastric cells. Fecal-oral, or oral-oral route is involved in childhood transmission. Moreover, H. pylori has a worldwide prevalence and is reported more frequently in developing countries (6). Besides to gastrointestinal diseases, evidence suggests H. pylori involvement in ITP (7, 8). Host antibodies against cytotoxin-associated gene A (CagA), which is a virulence factor of H. pylori can increase the rate of platelet clearance due to mimicry between CagA and platelet associated IgG (9, 10). In addition to specific antibodies, downregulation of monocyte FcγR receptor may shift the balance toward the increased phagocytic activity of monocytes, which may ultimately lead to thrombocytopenia (11). Various antibiotics and proton pump inhibitors (PPI) are widely used for the treatment of H. pylori (12). Several reports have shown that H. pylori eradication can increase platelet count in adult ITP (10-13).
2. Objectives
Of note, clinical trials have not yielded consistent findings. This was the purpose of our investigation. We have designed a randomized controlled study to explore the impact of H. pylori eradication on platelet count in Iranian children with acute ITP.
3. Methods
3.1. Study Design
Thirty children with a diagnosis of acute ITP and H. pylori infection were studied. They were new cases. This randomized, open-label, and parallel-group study was carried out in Amir-Kabir Hospital, Arak, Iran in 2018. We used a simple randomization method. Sealed envelopes with an enclosed assignment were used for allocation concealment. Normal health status, normal size of the liver and spleen, and negative results for bone marrow aspiration, hepatitis B, hepatitis C, and HIV were used for the diagnosis of ITP. Also, H. pylori stool antigen (HpSA) test was used for the diagnosis of H. pylori infection. The patients were randomly allocated 1:1 to standard ITP and H. pylori treatments or only standard ITP treatment. So ITP was treated with 2 mg/kg oral prednisolone for 2 weeks and a single dose of Intravenous Immunoglobulin (IVIG) (1 g/kg infused for 8 hours). The H. pylori eradication therapy was 1 mg/kg omeprazole for two weeks, 7.5 mg/kg clarithromycin twice a day for 10 days, and 50 mg/kg amoxicillin twice a day for 10 days. The studied subjects were followed for 6 months. The local Ethics Committee approved the study and informed consent was taken from the parent(s) or by the legal representative prior to trial participation. The clinical trial registry number was IRCT20141209020258N68.
The exclusion criteria were severe hepatic disorders, renal failure, positive Coombs test, infectious diseases, malignancies, receiving immunosuppressant and PPI, a history of H. pylori infection, and autoimmune diseases. Safety, lost to follow-up, and voluntary discontinuation were also considered for excluding the patients.
3.2. Efficacy Assessment
Platelet count was performed at baseline, 1week, 1month, 2 months, 3 months, and 6 months of the follow-up. Responses to ITP therapy during 6 months of follow up were defined as follows: if the platelet count was greater than 100 × 109/L it was defined as complete response, if it was from 10 × 109/L to 100 × 109/L it was defined as partial response, and if the platelet count was less than 10 × 109/L it was defined as no response. The rate of response was measured until the follow-up termination. Success in H. pylori eradication was evaluated by H. pylori stool antigen test (HpSA) after 6 weeks of the treatment termination.
3.3. Safety Assessment
Physical examination and cell blood count were monitored at baseline and 3, 7, and 14 days of the treatment. For the evaluation of untoward effects, patients were monitored for allergy, hemolytic anemia, and fever.
3.4. Data Analysis
The sample size was calculated according to Suzuki’s study (14). The total number of 30 patients was calculated for randomization with an α of 0.05 and β of 0.2. Data are shown in mean ± SD. The relationship between variables was tested by χ2 test and Fisher’s exact test. The differences between the two studied groups were analyzed by t-test. Changes in platelet counts were tested with repeated-measures ANOVA. P < 0.05 was statistically considered a significant difference. Analyses were carried out using SPSS software (version 18.0, Chicago, IL, US).
4. Results
4.1. Baseline Characteristics
Of the 30 children, 2 patients did not enter the randomized treatment. A total of 28 patients with the diagnosis of acute ITP and H. pylori infection were studied between March 2018 and August 2018. The study flowchart is shown in Figure 1. Clinical characteristics of the patients are shown in Table 1. The mean age was 11 years with the majority of females (67.9 % vs. 32.1 %). No significant differences were observed in the baseline characteristics of the studied children.
| Characteristics | ITP and H. pylori Therapy, N = 14 | ITP Therapy, N = 14 | P Value |
|---|---|---|---|
| Age, y | 6.3 ± 1.3 | 5.9 ± 1.1 | 0.3 |
| Age (range) | 2 - 14 | 2 - 13 | 0.1 |
| Female | 9 (64.2) | 10 (71.4) | 0.3 |
| Male | 5 (35.8) | 4 (28.6) | 0.5 |
| Hemoglobin, g/L | 107 ± 8.6 | 108 ± 8.7 | 0.3 |
| Total WBC count, 109/L | 7.9 ± 3.24 | 7.8 ± 2.1 | 0.8 |
| Platelets, 109/L | 16930 ± 1044 | 15000 ± 3800 | 0.7 |
Consort diagram detailing the study subjects

4.2. Efficacy
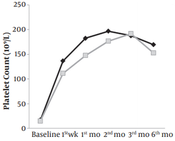
At baseline, the mean platelet count was 16.93 ± 10.1 (109/L) in the ITP and H. pylori therapy group, and15.0 ± 3.8 (109/L) in the ITP therapy group. After one week of the treatment, mean platelet counts increased to 136.5 ± 55.20 and 124.0 ± 45.70 in the two groups, respectively (109/L). Mean platelet counts at different time intervals are presented in Figure 2. The between-group differences were not statistically significant.
Effects of ITP therapy combined with H. pylori therapy and ITP therapy alone on platelet count over 6 months in 28 children

4.3. Rate of Response
After 6 months of follow up, from a total of 14 patients who received ITP and H. pylori treatments, 9 (64.2 %) children had a complete response, and 5 (35.7%) children had partial response. In 14 patients who only received ITP treatment, 9 (64.2 %) children had a complete response, and 5 (35.7%) children had a partial response. H. pylori eradication was successful in 12 (85%) children.
4.4. Adverse Effects
As presented in Figure 3, fever was reported in both groups. Mild gastrointestinal symptoms were more frequent in children who received H. pylori eradication therapies (P < 0.01). No significant drop was noted in hemoglobin levels at different time intervals. No participant died and no participant was withdrawn due to severe adverse effects.
Untoward effects of the treatments: fever, GI upset and chills. Fever was the most common reported side effects of both treatments. * P < 0.05, **P < 0.01.

5. Discussion
To the best of our knowledge, this is the first randomized controlled study, investigating the association between H. pylori eradication and platelet count in children with acute ITP. Our study confirms that H. pylori eradication did not significantly affect the platelet count compared to the standard ITP therapy. In this report, we show that standard ITP treatment increases platelet count compared to baseline values. Of note, our 6-month follow-up demonstrated that adding H. pylori therapy was not effective to improve the thrombocytopenic status. The association between ITP and H. pylori was first reported in Italian adult patients in whom H. pylori eradication was associated with a significant increase in platelet count in most of ITP patients (10). It should be noted that patients underwent long-term follow-up particularly in subjects who were older. So far, several prospective and retrospective studies have been conducted to study the relationship between pediatric ITP and H. pylori (12, 15, 16). Some of them have reported positive effects, while some of them have not. Of note, randomized controlled trials are very scarce. The last guideline of the American Society of Hematology (ASH 2011) has recommended the investigation for H. pylori in the work-up of children with ITP (17). However, the current ASH guideline is widely based on retrospective and prospective studies due to very limited randomized trials. Treepongkanura’s study was the first randomized controlled trial conducted in patients with chronic pediatric ITP who were infected with H. pylori (18). The study showed no impact of H. pylori eradication on platelet count in 16 children from 4 to 18 years old in Thailand. In comparison to this study, we included more patients with acute ITP and we had a different design. However, we had similar findings albeit in acute ITP. The second randomized controlled trial in this field is Brito’ study, which was done on 22 H. pylori-infected children and adolescents in Brazil (19). This trial showed that H. pylori eradication is associated with a platelet increase in the selected patients. Compared to our work, the studied children had a greater mean age and age range. In addition, Brito et al.’ (19)study was focused on chronic ITP patients. However, children up to 15 years with acute ITP were included in the current work. In contrast to their findings, we found no positive effect of H. pylori eradication on platelet count. Moreover, a work by Russo on 37 children with chronic ITP proved helpful effects of triple eradication therapy on platelet counts (20). It is noteworthy that 2 studies by Loffredo et al. and Yetgin et al. did not find any positive impact of eradication therapy on platelet count in chronic pediatric ITP (21, 22). Based on available findings, it seems that there are some contradictory findings against the linkage between H. pylori eradication and platelet number in childhood ITP. Our literature review found no study about acute ITP and H. pylori in children. Geographical differences in the prevalence of H. pylori, genetic variations in H. pylori, different lines of treatments, and various methods for the assessment may explain the lack of global consensus on ITP and H. pylori relationship. A recent study by Kim et al. showed that there are still some questions regarding ITP and H. pylori and more investigations and clinical trials are still recommended (23). At present, there is no report about H. pylori prevalence in the whole Iranian children. Jafar et al.’s study showed that the prevalence of H. pylori is 65% in children living in the west of Iran (24). Another work reported a very high prevalence of H. pylori in the south of Iran (25).
5.1. Study Limitations
Owing to the low prevalence of H. pylori, we could not carry out a larger trial. In addition, ITP is not very prevalent in children; therefore, it is difficult to apply a large randomized controlled trial to children with ITP who suffer from confirmed H. pylori infection.
5.2. Conclusions
It is suggested that H. pylori eradication did not significantly affect the platelet count compared to the standard ITP therapy in children with acute ITP. Further investigations are recommended to explore the mechanisms underlying H. pylori-associated ITP.
Acknowledgements
References
-
1.
Chong BH, Ho SJ. Autoimmune thrombocytopenia. J Thromb Haemost. 2005;3(8):1763-72. [PubMed ID: 16102043]. https://doi.org/10.1111/j.1538-7836.2005.01376.x.
-
2.
Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: Pathogenic and clinical diversity. Blood. 2009;113(26):6511-21. [PubMed ID: 19395674]. [PubMed Central ID: PMC2710913]. https://doi.org/10.1182/blood-2009-01-129155.
-
3.
Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346(13):995-1008. [PubMed ID: 11919310]. https://doi.org/10.1056/NEJMra010501.
-
4.
Lilleyman J. Medical nemesis and childhood ITP. Br J Haematol. 2003;123(4):586-9. [PubMed ID: 14616960]. https://doi.org/10.1046/j.1365-2141.2003.04656.x.
-
5.
Eghbali A, Azadmanesh P, Bagheri B, Taherahmadi H, Sadeghi Sedeh B. Comparison between IV immune globulin (IVIG) and anti-D globulin for treatment of immune thrombocytopenia: A randomized open-label study. Fundam Clin Pharmacol. 2016;30(4):385-9. [PubMed ID: 26991138]. https://doi.org/10.1111/fcp.12198.
-
6.
Mentis A, Lehours P, Megraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2015;20 Suppl 1:1-7. [PubMed ID: 26372818]. https://doi.org/10.1111/hel.12250.
-
7.
Franchini M, Veneri D. Helicobacter pylori infection and immune thrombocytopenic purpura: An update. Helicobacter. 2004;9(4):342-6. [PubMed ID: 15270749]. https://doi.org/10.1111/j.1083-4389.2004.00238.x.
-
8.
Emilia G, Luppi M, Torelli G. Infectious agents and human immune diseases: Lessons from Helicobacter pylori. Am J Med. 2005;118(4):420-1. [PubMed ID: 15808141]. https://doi.org/10.1016/j.amjmed.2005.02.003.
-
9.
Kodama M, Kitadai Y, Ito M, Kai H, Masuda H, Tanaka S, et al. Immune response to CagA protein is associated with improved platelet count after Helicobacter pylori eradication in patients with idiopathic thrombocytopenic purpura. Helicobacter. 2007;12(1):36-42. [PubMed ID: 17241299]. https://doi.org/10.1111/j.1523-5378.2007.00477.x.
-
10.
Takahashi T, Yujiri T, Shinohara K, Inoue Y, Sato Y, Fujii Y, et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124(1):91-6. [PubMed ID: 14675413]. https://doi.org/10.1046/j.1365-2141.2003.04735.x.
-
11.
Asahi A, Nishimoto T, Okazaki Y, Suzuki H, Masaoka T, Kawakami Y, et al. Helicobacter pylori eradication shifts monocyte Fcgamma receptor balance toward inhibitory FcgammaRIIB in immune thrombocytopenic purpura patients. J Clin Invest. 2008;118(8):2939-49. [PubMed ID: 18654664]. [PubMed Central ID: PMC2483681]. https://doi.org/10.1172/JCI34496.
-
12.
Inaba T, Mizuno M, Take S, Suwaki K, Honda T, Kawai K, et al. Eradication of Helicobacter pylori increases platelet count in patients with idiopathic thrombocytopenic purpura in Japan. Eur J Clin Invest. 2005;35(3):214-9. [PubMed ID: 15733077]. https://doi.org/10.1111/j.1365-2362.2005.01471.x.
-
13.
Michel M, Khellaf M, Desforges L, Lee K, Schaeffer A, Godeau B, et al. Autoimmune thrombocytopenic Purpura and Helicobacter pylori infection. Arch Intern Med. 2002;162(9):1033-6. [PubMed ID: 11996614]. https://doi.org/10.1001/archinte.162.9.1033.
-
14.
Suzuki T, Matsushima M, Masui A, Watanabe K, Takagi A, Ogawa Y, et al. Effect of Helicobacter pylori eradication in patients with chronic idiopathic thrombocytopenic purpura-a randomized controlled trial. Am J Gastroenterol. 2005;100(6):1265-70. [PubMed ID: 15929755]. https://doi.org/10.1111/j.1572-0241.2005.41641.x.
-
15.
Veneri D, Krampera M, Franchini M. High prevalence of sustained remission of idiopathic thrombocytopenic purpura after Helicobacter pylori eradication: A long-term follow-up study. Platelets. 2005;16(2):117-9. [PubMed ID: 15823868]. https://doi.org/10.1080/09537100400015153.
-
16.
Tag HS, Lee HS, Jung SH, Kim BK, Kim SB, Lee A, et al. Effects of Helicobacter pylori eradication in patients with immune thrombocytopenic purpura. Korean J Hematol. 2010;45(2):127-32. [PubMed ID: 21120192]. [PubMed Central ID: PMC2983021]. https://doi.org/10.5045/kjh.2010.45.2.127.
-
17.
Neunert C, Lim W, Crowther M, Cohen A, Solberg LJ, Crowther MA, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190-207. [PubMed ID: 21325604]. https://doi.org/10.1182/blood-2010-08-302984.
-
18.
Treepongkaruna S, Sirachainan N, Kanjanapongkul S, Winaichatsak A, Sirithorn S, Sumritsopak R, et al. Absence of platelet recovery following Helicobacter pylori eradication in childhood chronic idiopathic thrombocytopenic purpura: A multi-center randomized controlled trial. Pediatr Blood Cancer. 2009;53(1):72-7. [PubMed ID: 19301380]. https://doi.org/10.1002/pbc.21991.
-
19.
Brito HS, Braga JA, Loggetto SR, Machado RS, Granato CF, Kawakami E. Helicobacter pylori infection & immune thrombocytopenic purpura in children and adolescents: A randomized controlled trial. Platelets. 2015;26(4):336-41. [PubMed ID: 24832381]. https://doi.org/10.3109/09537104.2014.911836.
-
20.
Russo G, Miraglia V, Branciforte F, Matarese SM, Zecca M, Bisogno G, et al. Effect of eradication of Helicobacter pylori in children with chronic immune thrombocytopenia: A prospective, controlled, multicenter study. Pediatr Blood Cancer. 2011;56(2):273-8. [PubMed ID: 20830773]. https://doi.org/10.1002/pbc.22770.
-
21.
Loffredo G, Marzano MG, Migliorati R, Miele E, Menna F, Poggi V, et al. The relationship between immune thrombocytopenic purpura and Helicobacter pylori infection in children: where is the truth? Eur J Pediatr. 2007;166(10):1067-8. [PubMed ID: 17136353]. https://doi.org/10.1007/s00431-006-0344-4.
-
22.
Yetgin S, Demir H, Arslan D, Unal S, Kocak N. Autoimmune thrombocytopenic purpura and Helicobacter pylori infection effectivity during childhood. Am J Hematol. 2005;78(4):318. [PubMed ID: 15795919]. https://doi.org/10.1002/ajh.20302.
-
23.
Kim BJ, Kim HS, Jang HJ, Kim JH. Helicobacter pylori eradication in idiopathic thrombocytopenic purpura: A meta-analysis of randomized trials. Gastroenterol Res Pract. 2018;2018:6090878. [PubMed ID: 30402091]. [PubMed Central ID: PMC6198559]. https://doi.org/10.1155/2018/6090878.
-
24.
Jafar S, Jalil A, Soheila N, Sirous S. Prevalence of helicobacter pylori infection in children, a population-based cross-sectional study in west iran. Iran J Pediatr. 2013;23(1):13-8. [PubMed ID: 23550042]. [PubMed Central ID: PMC3574986].
-
25.
Alborzi A, Soltani J, Pourabbas B, Oboodi B, Haghighat M, Hayati M, et al. Prevalence of Helicobacter pylori infection in children (south of Iran). Diagn Microbiol Infect Dis. 2006;54(4):259-61. [PubMed ID: 16466888]. https://doi.org/10.1016/j.diagmicrobio.2005.10.012.