Abstract
Introduction:
Organ transplant recipients might be more likely to develop COVID-19, as they receive long-term immunosuppressives and have comorbidities.Case Presentation:
Herein, we reported the case of a 32-year-old man with unilateral lung transplantation due to unclassifiable lung fibrosis on pathologic evaluation who presented with cough, fever, and headache. After evaluation with RT-PCR test and chest CT scan, COVID-19 in the previously transplanted lung was diagnosed. However, the other non-transplanted fibrotic lung was not involved.Conclusions:
Lack of COVID-19 involvement in the fibrotic lung tissue in our case without any other risk factors might be related to the fact that the lung with underlying diseases was less susceptible to COVID-19 as unhealthy lungs have lower ACE2 receptors, or it might be related to genetic differences between the donor and recipient.Keywords
1. Introduction
In December 2019, the novel coronavirus (SARS-CoV-2) that led to coronavirus disease 2019 (COVID-19) was first identified in Wuhan, China (1). Solid-organ transplant recipients might be more likely to develop COVID-19, as they receive long-term immunosuppressives and have comorbidities (2). A number of cases have been published on COVID-19 pneumonia in solid-organ transplant recipients (3-6). Verleden et al. showed a high rate of hospital admission (eight of ten), but with low mortality rate (one of ten), in patients with lung transplantation who developed COVID-19. Symptoms in their patients were quite comparable to a non-transplanted population and was nearly mild to moderate (6). Bilateral ground glass opacities were seen in six patients, whereas the remaining four cases had no radiologic abnormalities (6). However, in the study by Saez-Gimenez, among 44 lung transplanted cases, the mortality (39%) and morbidity rates were quite high. Acute parenchymal abnormalities were found in 73% of cases on chest radiography, and 84% of the patients required some degree of respiratory support, and 30% were admitted to intermediate or intensive critical care units (7).
So far, COVID-19 infection in the unilateral transplanted lung without involving the other non-transplanted lung has not been reported.
2. Case Presentation
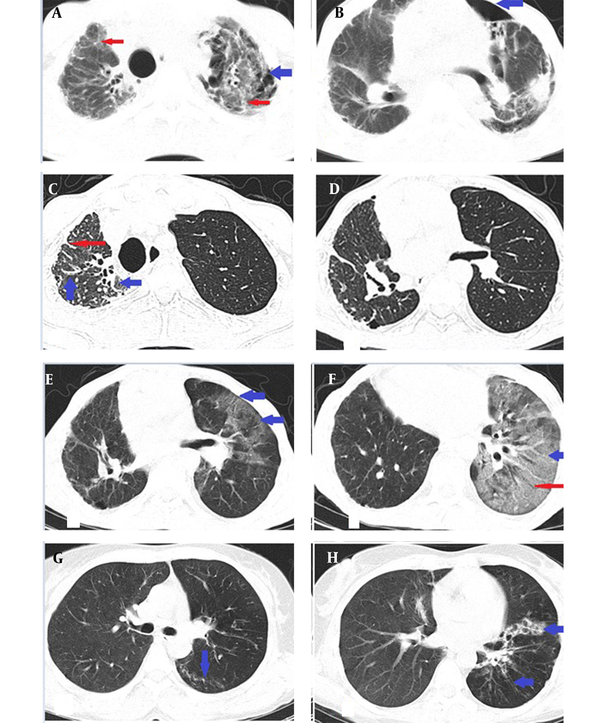
A 32-year-old man underwent unilateral left-side lung transplantation due to unclassifiable lung fibrosis on pathologic evaluation three years ago, and since then, he had received tacrolimus, mycophenolate mofetil, and prednisolone. He visited the hospital with fever, cough, headache, nausea, vomiting, and myalgia, which had started about four days before admission. General physical examination included an oral temperature of 38°C, a heart rate of 95 beats per minute, a respiratory rate of 20 breaths per minute, and a blood pressure of 90/60 mmHg. Arterial blood oxygen saturation was 87%, and RT-PCR test for SARS-COV-2 was positive. The initial leukocyte count was 800 μL (Lymph: 6.2%). On the chest CT scan, patchy ground glass opacities, crazy paving pattern, and traction bronchiectasis were present in the transplanted left lung. However, no new findings other than signs of old fibrosis, such as reticulation and traction bronchiectasis, were detected in the right lung (Figure 1).
Computed tomography scan manifestation of lung fibrosis and COVID-19 in a 32-year-old man with unilateral lung transplantation due to unclassifiable lung fibrosis. (A) Initial CT scan, before transplantation: Bilateral lungs showed reticulation (thin red arrows) with left-side cyst formation (thick blue arrow). Pathologic evaluation confirmed fibrosis suggestive of idiopathic end-stage lung disease. (B) Same CT scan as (A), left side superimposed pneumothorax (blue arrow) was present. (C, D) The patient underwent unilateral left side lung transplantation three years ago. Right lung reticulation (thin red arrow) and traction bronchiectasis (thick blue arrow) suggestive of fibrosis were present. (E) Chest CT scan showed patchy ground glass opacities due to involvement with COVID-19 (blue arrows) in the left transplanted lung with no involvement of the right fibrotic non-transplanted lung. (F) Same CT scan as in (E) with left lower lobe crazy paving (thick blue arrow) and traction bronchiectasis (thin red arrow). (G) CT scan of the patient 10 days after the previous CT (E, F) with significant resolution of the disease and linear opacity (blue arrow) of the left lung. (H) Same CT scan as in F shows significant resolution of the left ground glass opacities. Traction bronchiectasis (blue arrows) is still visible.
During hospitalization, he received famotidine 20 mg twice a day, remdesivir with a loading dose of 200 mg, a maintenance dose of 100 mg intravenously every 24 hours for a five-day course, dexamethasone 4 mg twice a day, granulocyte colony stimulating factor (GCSF) 300 mcg subcutaneously daily, and enoxaparin 40 mg subcutaneously daily.
On day 11 of admission, he recovered and was released from the hospital. Lung opacities on the chest CT were resolved, and only linear opacity and traction bronchiectasis (Figure 1) remained in the left lower lung lobe. Leukocyte count elevated after treatment (10000 μL, Lymph: 28%).
3. Conclusions
Our young patient experienced COVID-19 pneumonia in the transplanted lung without affecting the non-transplanted fibrotic lung, which might be related to genetic differences between the donor’s and recipient’s lungs (8, 9).
It was demonstrated that a spike (S) protein of SARS-CoV-2 specifically detects the host angiotensin-converting enzyme 2 (ACE2) receptor to enter and replicate the virus into the host cells. In particular, the ACE2 receptor is expressed in type 2 alveolar epithelial cells in the lungs, heart, kidney, and gastrointestinal tract. However, the large surface area in the lungs is considered the main host for virus replication (10). Alveolar epithelial type II cells, as the main target of ACE2 in the lung, grow rapidly in pulmonary fibrosis, but ACE-2 receptor reduced (11). In addition, the distribution of ACE2 in different organs was significantly associated with the clinical symptoms of SARS-CoV-2 infection. Different host susceptibilities is a probable main factor for developing organ failure and disease severity (12).
Although the exact mechanisms of SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) and progression to the severe disease are unclear, different mechanisms of lung injury were previously suggested, including direct damage due to viral replication, local inflammatory response, cytokine storm, and the systemic inflammatory response (13, 14). Some cytokines such as IL-2, IL-8, IL-10, and IL-12 were found to be increased in pulmonary fibrosis, but IFN-γ level decreased in these patients (15). M-CSF, IL-10, IFN-α2, IL-17, IL-4, IP-10, IL-7, IL-1ra, G-CSF, IL-12, IFN-γ, IL-1α, IL-2, HGF, and PDGF-BB were significantly correlated with lung-injury Murray score and could predict severe COVID-19 (16).
Interestingly, patients with asthma were not more likely to develop severe COVID-19 pneumonia requiring hospitalization (17). Lack of COVID-19 involvement in the fibrotic lung tissue in our patient and no increased risk of some pulmonary diseases such as asthma for COVID-19 infection showed that underlying lung diseases might be less likely to develop COVID-19 as unhealthy lungs have lower ACE2 receptors.
In sum, genetic differences and lung vulnerability to COVID-19 due to different ACE-2 distributions between donor’s healthy lung and our patient’s fibrotic lung might be the cause of COVID-19 involvement in the donor’s lung.
References
-
1.
Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev. 2020;7(6):1012-23. [PubMed ID: 34676127]. [PubMed Central ID: PMC7107875]. https://doi.org/10.1093/nsr/nwaa036.
-
2.
Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076-82. [PubMed ID: 32354637]. [PubMed Central ID: PMC7142878]. https://doi.org/10.1016/j.kint.2020.03.018.
-
3.
Aigner C, Dittmer U, Kamler M, Collaud S, Taube C. COVID-19 in a lung transplant recipient. J Heart Lung Transplant. 2020;39(6):610-1. [PubMed ID: 32340870]. [PubMed Central ID: PMC7152862]. https://doi.org/10.1016/j.healun.2020.04.004.
-
4.
Arpali E, Akyollu B, Yelken B, Tekin S, Turkmen A, Kocak B. Case report: A kidney transplant patient with mild COVID-19. Transpl Infect Dis. 2020;22(4). e13296. [PubMed ID: 32301198]. [PubMed Central ID: PMC7235513]. https://doi.org/10.1111/tid.13296.
-
5.
Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532-3. https://doi.org/10.1016/s2468-1253(20)30116-3.
-
6.
Verleden GM, Godinas L, Lorent N, Van Bleyenbergh P, Dupont L, Delcroix M, et al. COVID-19 in lung transplant patients: A case series. Am J Transplant. 2020;20(11):3234-8. [PubMed ID: 32659857]. [PubMed Central ID: PMC7404955]. https://doi.org/10.1111/ajt.16212.
-
7.
Saez-Gimenez B, Berastegui C, Barrecheguren M, Revilla-Lopez E, Los Arcos I, Alonso R, et al. COVID-19 in lung transplant recipients: A multicenter study. Am J Transplant. 2021;21(5):1816-24. [PubMed ID: 33089648]. https://doi.org/10.1111/ajt.16364.
-
8.
Severe Covid Gwas Group, Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med. 2020;383(16):1522-34. [PubMed ID: 32558485]. [PubMed Central ID: PMC7315890]. https://doi.org/10.1056/NEJMoa2020283.
-
9.
Hou Y, Zhao J, Martin W, Kallianpur A, Chung MK, Jehi L, et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020;18(1):216. [PubMed ID: 32664879]. [PubMed Central ID: PMC7360473]. https://doi.org/10.1186/s12916-020-01673-z.
-
10.
Wan Y, Shang J, Graham R, Baric RS, Li F, Gallagher T. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. Virol J. 2020;94(7). https://doi.org/10.1128/jvi.00127-20.
-
11.
Uhal BD, Dang M, Dang V, Llatos R, Cano E, Abdul-Hafez A, et al. Cell cycle dependence of ACE-2 explains downregulation in idiopathic pulmonary fibrosis. Eur Respir J. 2013;42(1):198-210. [PubMed ID: 23100504]. https://doi.org/10.1183/09031936.00015612.
-
12.
Babadaei MMN, Hasan A, Bloukh SH, Edis Z, Sharifi M, Kachooei E, et al. The expression level of angiotensin-converting enzyme 2 determines the severity of COVID-19: lung and heart tissue as targets. J Biomol Struct Dyn. 2021;39(10):3780-6. [PubMed ID: 32397951]. [PubMed Central ID: PMC7284141]. https://doi.org/10.1080/07391102.2020.1767211.
-
13.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-4. https://doi.org/10.1016/s0140-6736(20)30628-0.
-
14.
Torres Acosta MA, Singer BD. Pathogenesis of COVID-19-induced ARDS: implications for an ageing population. Eur Respir J. 2020;56(3). [PubMed ID: 32747391]. [PubMed Central ID: PMC7397945]. https://doi.org/10.1183/13993003.02049-2020.
-
15.
Tsoutsou PG, Gourgoulianis KI, Petinaki E, Germenis A, Tsoutsou AG, Mpaka M, et al. Cytokine levels in the sera of patients with idiopathic pulmonary fibrosis. Respir Med. 2006;100(5):938-45. [PubMed ID: 16236490]. https://doi.org/10.1016/j.rmed.2005.06.016.
-
16.
Liu Y, Zhang C, Huang F, Yang Y, Wang F, Yuan J, et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl Sci Rev. 2020;7(6):1003-11. [PubMed ID: 34676126]. [PubMed Central ID: PMC7107806]. https://doi.org/10.1093/nsr/nwaa037.
-
17.
Beurnier A, Jutant EM, Jevnikar M, Boucly A, Pichon J, Preda M, et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020;56(5). [PubMed ID: 32732333]. [PubMed Central ID: PMC7397950]. https://doi.org/10.1183/13993003.01875-2020.

.png)
