1. Background
Coronavirus disease 2019 (COVID-19) is a contagious infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This novel RNA virus was first identified in pneumonia patients with a cluster of acute respiratory disease signs and symptoms in Wuhan, China, in 2019. COVID-19, declared a global pandemic by the World Health Organization (WHO) in March 2020, has spread rapidly worldwide (1).
SARS-CoV-2 is primarily transmitted between individuals through respiratory droplets and close contact. This disease has a broad clinical spectrum, ranging from asymptomatic cases to mild (e.g., fever, cough, fatigue, dyspnea, anosmia, and ageusia) and severe or critical (e.g., septic shock, multiorgan failure, and acute respiratory distress syndrome) symptoms (1, 2). Considering the pandemic status of COVID-19 and global concerns, various diagnostic tests have been developed to identify the patients. A rapid and accurate diagnosis of COVID-19 is essential not only for its clinical management but also for public health control and development of therapies. Traditional methods, such as cell culture and electron microscopy, are the gold standard methods for detecting new viruses in laboratories; however, they cannot be applied as routine tests.
Real-time reverse transcription-polymerase chain reaction (RT-PCR) of specimens collected from nasopharyngeal and/or oropharyngeal swabs has shown high sensitivity and specificity in detecting COVID-19; therefore, it has become the gold standard for COVID-19 diagnosis. Moreover, a rapid diagnostic test (RDT) has been developed for COVID-19, based on either a serological test of peripheral blood for detecting the patient's immune response to infection or detection of viral antigens in a nasopharyngeal swab. Radiological imaging modalities, such as computed tomography (CT) scans, can also provide additional information about radiological changes in the patient's lungs (3, 4).
In addition to the mentioned diagnostic methods, a diagnostic tool is currently being developed for COVID-19 by detecting the presence of volatile organic compounds (VOCs) from exhaled breath. Generally, exhaled breath contains thousands of metabolites and VOCs from the airway and internal organ systems, containing various microbiomes. In the form of commensal and pathogenic bacteria and viruses, microbiomes can produce different classes of VOCs; some can even act as biomarkers for diagnosing and monitoring various diseases (5).
Breath gas analysis of VOCs has become a growing field of research in recent years. During blood gas exchange in the pulmonary alveoli, carbon dioxide (CO2) is released, and oxygen (O2) is taken up from the inhaled air. This exchange also applies to volatile metabolites directly produced in the body or the lungs. Based on this mechanism, researchers are trying to detect volatile biomarkers in exhaled breath, which can indicate a disease or response to pharmacological treatments (6).
According to some studies, it is possible to directly detect SARS-CoV-2 from exhaled breath only by using specific devices that can collect and condense exhaled breath for several minutes. The virus can be extracted following the standard PCR method using this condensate. It has been extensively reported that the virus triggers the cells to produce metabolites, causing VOCs to be exhaled. These VOCs can be the target of breath diagnostics and used to assess the health status of patients non-invasively (7).
In 2021, Grassin-Delyle et al. reported the detection of SARS-CoV-2 directly from the exhaled breath and cough of patients with acute respiratory disease. This study aimed to demonstrate the efficacy of face masks in preventing viral diffusion and also suggested the possibility of direct viral detection from breath. This approach has attracted significant attention to other viral diagnostics. Overall, developing a non-invasive test to detect COVID-19 can be a significant step in managing this pandemic. There is a high probability that this disease can be detected in exhaled breath. Besides, monitoring the impact of treatment or medications on exhaled breath seems highly relevant (8).
A study by Chen et al. found that COVID-19 patients, compared to the average population, had a higher level of VOCs in the form of ethyl butanoate and lower levels of butyraldehyde and isopropanol (9). Another study reported that the four most prominent VOCs in COVID-19 were methylpent-2-enal, 2,4-octadiene 1-chloroheptane, and nonanal, with typical concentrations of 10 to 250 ppb (8). So far, using VOCs as a diagnostic tool has been combined with technologies, such as artificial intelligence (AI), deep sensing algorithms (DSA), and machine learning, to produce more accurate diagnostic methods.
COVID-19 diagnosis from exhaled breath can be a potentially rapid, non-invasive, cost-effective, and simple diagnostic method (10). The presence of VOCs in exhaled breath occurs in the early stages of infection, leading to the immediate detection of COVID-19.
2. Objectives
So far, comprehensive reviews have been published on the potential of VOCs as chemical biomarkers for diagnostics (11). However, the accuracy of this method in diagnosing COVID-19 has yet to be widely known. Therefore, this evidence-based study aimed to collect and examine existing studies on the accuracy of VOC detection in exhaled breath for diagnosis of COVID-19 and comparison of this method with RT-PCR.
2.1. Clinical Question
With this background in mind, the clinical question of this study was formulated, as shown in Table 1.
| Parameters | Population | Intervention | Comparison | Outcomes |
|---|---|---|---|---|
| Patients indicated for COVID-19 testing | VOCs in exhaled breath | RT-PCR assay of nasopharyngeal and/or oropharyngeal swabs | COVID-19 diagnosis | |
| Clinical question | How accurate is the detection of VOCs from exhaled breath compared to RT-PCR in diagnosis of COVID-19? | |||
| Question type | Diagnosis | |||
| Study type | Meta-analysis, systematic review of cross-sectional studies, and cross-sectional | |||
Abbreviations: VOCs, volatile organic compounds; RT-PCR, reverse transcription-polymerase chain reaction.
3. Methods
3.1. Search Strategy
A literature search was conducted in five databases, including PubMed, Cochrane Library, ProQuest, EBSCOHost, and Scopus, in April 2021. The search was carried out in the titles and abstracts of articles using the following keywords and their equivalent terms, combined with the Boolean operators (OR and AND): "volatile organic compounds," "polymerase chain reaction," and "COVID-19." The search queries from all databases and the results are presented in Table 2. Along with the search in the databases, a literature search was also carried out manually.
| Databases | Search Queries | Hits | Selected Articles |
|---|---|---|---|
| PubMed | ("Volatile organic compound" [Title/Abstract] OR "VOC" [Title/Abstract]) AND ("polymerase chain reaction" [Title/Abstract] OR "PCR" [Title/Abstract] OR "RT-PCR" [Title/Abstract]) AND ("COVID-19" [Title/Abstract] OR "SARS-CoV-2" [Title/Abstract] OR "coronavirus" [Title/Abstract]) | 10 | 2 |
| Cochrane Library | ((Volatile organic compound):ti,ab,kw OR (VOC):ti,ab,kw) AND (("polymerase chain reaction"):ti,ab,kw OR (PCR):ti,ab,kw OR (RT-PCR):ti,ab,kw) AND ((COVID-19):ti,ab,kw OR (SARS-CoV-2):ti,ab,kw OR (coronavirus):ti,ab,kw) | 1 | 0 |
| ProQuest | (("Volatile organic compound" OR "VOC") AND ("polymerase chain reaction" OR "PCR" OR "RT-PCR") AND ("COVID-19" OR "SARS-CoV-2" OR "coronavirus"):ab) | 7 | 0 |
| EBSCOHost | (("Volatile organic compound" OR "VOC") AND ("polymerase chain reaction" OR "PCR" OR "RT-PCR") AND ("COVID-19" OR "SARS-CoV-2" OR "coronavirus"):ab) | 7 | 0 |
| Scopus | (("Volatile organic compound" OR "VOC") AND ("polymerase chain reaction" OR "PCR" OR "RT-PCR") AND ("COVID-19" OR "SARS-CoV-2" OR "coronavirus"):ti,ab,kw) | 12 | 0 |
3.2. Search Selection
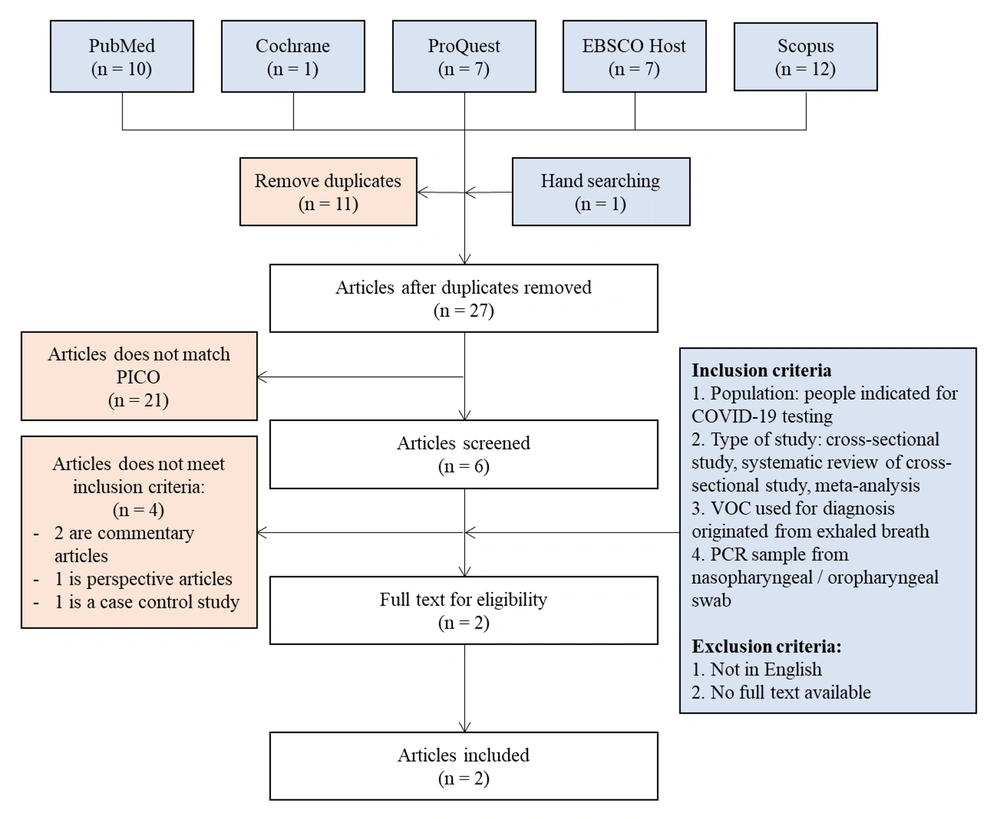
The search results of all five databases, besides the results of hand searching, yielded 38 articles. After preliminary screening for duplicates, 11 articles were excluded, and 27 remained in the review. Next, the titles and abstracts of the articles were screened. Eleven articles that did not match the Population, Intervention, Control, and Outcomes (PICO) format were excluded, leaving six articles. The full texts of the remaining articles were then analyzed according to the eligibility criteria. Finally, two articles were included in this review.
The eligibility criteria for the articles were as follows: (1) evaluation of populations indicated for COVID-19 testing; (2) cross-sectional studies, systematic reviews of cross-sectional studies, or meta-analyses; (3) use of VOCs from exhaled breath for diagnosis; and (4) collection of a PCR sample from the nasopharyngeal/oropharyngeal swab. On the other hand, articles that did not meet the inclusion criteria, were not written in English, and were not available in full text were excluded. The flowchart of the study selection is presented in Figure 1.
4. Results
4.1. Summary of Articles
Two studies conducted by Wintjens et al. (12) and Ruszkiewicz et al. (13) were examined in this study. Ruszkiewicz et al.’s study consisted of two analyses in two locations with different samples and analyses. The level of evidence was determined based on the 2011 Oxford Center of Evidence-Based Medicine (CEBM) criteria. The summary of these two studies is presented in Table 3.
| Parameters | Wintjens et al. (12) | Ruszkiewicz et al. (13) |
|---|---|---|
| Title | “Applying the electronic nose for pre-operative SARS-CoV-2 screening” | “Diagnosis of COVID-19 by analysis of breath with gas chromatography ion mobility spectrometry: A feasibility study” |
| Study type | Cross-sectional | Cross-sectional |
| Level of evidence | 2 (CEBM, 2011) | 2 (CEBM, 2011) |
| Number of subjects | n = 219 | Edinburgh study: n = 25; Dortmund study: n = 65 |
| Population | Employees with symptoms of COVID-19 and confirmed COVID-19 patients in Maastricht University Medical Center (MUMC+, the Netherlands) | Edinburgh study: Patients with respiratory symptoms of COVID-19 presenting to the emergency department of the Royal Infirmary of Edinburgh, UK. Dortmund study: Outpatients or patients with respiratory symptoms presenting to the emergency department of Klinikum Dortmund, Germany. |
| Intervention | VOCs from exhaled breath detected with the Aeonose device (with a metal-oxide sensor) | VOCs from exhaled breath detected via GC-IMS |
| Comparison | RT-PCR assay of nasopharyngeal/oropharyngeal swabs | RT-PCR of nasopharyngeal/oropharyngeal swabs |
Abbreviations: GC-IMS, gas chromatography-ion mobility spectrometry; RT-PCR, real-time reverse transcription-polymerase chain reaction (RT-PCR).
4.2. Critical Appraisal
Three components of an article were investigated for the critical appraisal using the Oxford CEBM Critical Appraisal Tool: validity, importance, and applicability. The critical appraisal of studies included in this review is presented in the following tables (Tables 4 to 9).
| Validity Criteria | Wintjens et al. (12) | Ruszkiewicz et al. (13) |
|---|---|---|
| Is the diagnostic test evaluated in a representative spectrum of patients? | Yes, the population tested included patients indicated for COVID-19 testing who were consecutively admitted to a hospital. | Yes, the population tested included patients indicated for COVID-19 testing who were consecutively admitted to a hospital. |
| Is the reference standard applied regardless of the index test result? | Yes, the VOC breath test and RT-PCR were performed for all patients. | Yes, the VOC breath test and RT-PCR were performed for all patients. |
| Is there an independent, blind comparison between the index test and the gold standard of diagnosis? | No | No |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; LR (+), positive likelihood ratio; LR (-), negative likelihood ratio.
| Applicability Criteria | Wintjens et al. (12) | Ruszkiewicz et al. (13) |
|---|---|---|
| Are the test methods described in sufficient detail to allow replication? | Yes, the article presents a sufficient description of the test to allow its replication and interpretation of the results. | Yes, the article presents a comprehensive description of the test to allow its replication and interpretation of the results. |
The results of each study were entered into a 2 × 2 contingency table. As mentioned earlier, Ruszkiewicz et al. (13) conducted two analyses in different settings and reported different results; therefore, they were described separately in the table. Overall, three comparisons were made in terms of importance.
The results reported in these tables were then used to assess different aspects of importance.
5. Discussion
Generally, VOCs are the end products of carbohydrate and lipid metabolism, oxidative stress, and liver cytochrome p450 enzymes in human cells, besides aerobic and anaerobic fermentation processes by bacteria living in the gut microbiome (5). Under physiological conditions, various VOCs, such as acetate, propionate, short-chain fatty acids (SCFAs), alcohols, propanol, hydrocarbons, aldehydes, nitrogen, and sulfur-containing compounds, are exhaled from the human breath (14). However, many VOCs can act as biological markers for the detection of oxidative stress, inflammation, carcinogens, and microbial infections (15, 16).
In terms of COVID-19, a recent study by Chen et al. measured various VOCs from exhaled breath in COVID-19 patients and compared them with those of healthy controls and patients with a non-COVID respiratory infection or lung cancer. It was revealed that a VOC profile of lower butyraldehyde levels but higher ethyl butanoate levels corresponded to COVID-19 infection. In contrast, higher levels of butyraldehyde and ethyl butanoate probably resulted from infections caused by pathogens other than SARS-CoV-2 (9). Although this study had a small sample size, it could support the use of VOCs for the diagnosis of COVID-19.
Two studies were retrieved based on the literature search strategy and critically appraised. Both studies had a cross-sectional design, with a high level of evidence for diagnostic studies, according to the 2011 CEBM criteria (level 2). Also, the validity of diagnostic studies was appraised based on a representative spectrum of patients. The diagnostic test and reference standard were examined in all patients, and an independent, blind comparison was made between them. Both studies by Wintjens et al. (12) and Ruszkiewicz et al. (13) met the first two criteria, while there was no information regarding the independent, blind comparisons; however, it can be concluded that these studies are valid.
The critical aspects of the retrieved studies were appraised based on sensitivity, specificity, and predictive values. It should be noted that although in both studies, the diagnostic methods detected VOCs from exhaled breath, the devices used varied. In the study by Wintjens et al., an Aeonose device with a metal-oxide-based sensor was used for detecting VOCs in exhaled breath. The device showed 86% sensitivity with a negative predictive value (NPV) of 92%, while its specificity was 54% with the positive predictive value (PPV) of 40%. The high sensitivity and NPV implied that Aeonose was adequate in identifying people with COVID-19 and could be used for diagnostic triage to exclude a SARS-CoV-2 infection. However, the specificity and PPV were inappropriate; therefore, further investigation is required if an individual tests positive with this device.
Similar results were reported by Ruszkiewicz et al. (13) in Dortmund, Germany, as they reported high sensitivity (90%) with a high NPV (97.8%) and high specificity (80%) with a low PPV (45%) for their method. It can be concluded that the gas chromatography-ion migration spectroscopy (GC-IMS) method is also appropriate for excluding COVID-19, with fewer false-negative results, thereby making it a superior diagnostic triage tool. On the other hand, Ruszkiewicz et al. in Edinburgh showed a significantly lower NPV (66.7%) but a higher PPV (87.5%) compared to the other two studies. The sensitivity and specificity were also lower than those reported in the Dortmund study (82.4% and 75%, respectively). Overall, the results of their studies were not highly consistent, and their accuracy ranged from 62 to 82%.
In the study conducted by Ruszkiewicz et al., there is a possible bias from the patient's diet. The diet can influence exhaled VOCs and possibly produce confounding data and false positive results. However, in this study, the dietary factors were managed carefully (13). As for the study generated by Wintjens et al., the bias comes from using the RT-PCR test as a reference standard. As the RT-PCR procedure mainly has low sensitivity, the possibility of missing infected participants was high, resulting in an inaccurate study algorithm (12).
In terms of applicability, both studies described the tests in sufficient detail to allow replication. In the study by Wintjens et al., (12) Aeonose could be a rapid, low-cost, and non-invasive test for COVID-19; therefore, it was applicable in the triage of health facilities. Besides, Aeonose has been previously examined in Indonesia for the diagnosis of tuberculosis (17). GC-IMS is also widely used to detect VOCs in various respiratory diseases, such as acute respiratory distress syndrome (18).
5.1. Conclusions
Detection of VOCs from exhaled breath can be a rapid, cost-effective, and simple method for diagnosing COVID-19. However, the accuracy of this method still needs to be higher (62 - 82%), and the studies had a small sample size with inconsistent results. The tools used also varied and needed to be standardized. Although this method was proposed as a screening tool, further studies are required with a larger sample size and standardized equipment to obtain more accurate and consistent results.