Abstract
Keywords
1. Introduction
A variety of infectious diseases presented as undifferentiated febrile syndromes can mimic leptospirosis (1). It appears that this disease is not that common in kids in Iran, however unnecessary diagnostic and therapeutic steps may be taken if the possibility is neglected. Here, we introduce a case which could be wrongly diagnosed as systemic vasculitis with no obvious source of contamination.
2. Case Presentation
A thirteen-year-old girl from Dezfoul, Iran was referred to our hospital with history of eight days of high fever, headache, odynophagia, and diffuse abdominal and body pain especially limb pain. She had received one shot of benzathine penicillin without any improvement. After 4 days, she developed conjunctival congestion, lip desquamation and also erythematous rash on the cheeks, neck and upper parts of her chest. Physical exam on admission revealed an ill appearance with vital signs as follows: blood pressure = 80/50 mmHg, pulse rate = 118/min, respiratory rate = 30/min and temperature = 39°C. There was bilateral non-purulent conjunctivitis, pharyngeal erythema without exudation, small red papules on the cheeks, neck and upper chest with sparing the nasolabial folds. A 2/6 systolic murmur was also evident. The rest of the examination was normal, except for bilateral mild edema on the lower legs. No history of travel from her city, swimming or animal contact or consumption of raw dairy product was present. She was a smart student with no previous history of any significant medical problem. In addition, there was no history of weight loss, drug exposure or rheumatologic diseases in her family. To rule out viral and bacterial causes of such a picture some preliminary investigations were done. The results are shown in Table 1.
Results of Laboratory Tests
| Requested tests | Results |
|---|---|
| CBC | WBC=11.9×10³ (segmented: 69%, band: 18%, lymphocyte: 10%, monocyte: 3%) |
| Sedimentation rate | 94 mm/1th hour |
| Platelet count | 238,000/mm³ on first exam, then increased to 640,000/mm³ |
| Biochemistry | Na, K, Ca, urinary creatinin and calcium as well as 24 hours urinary protein were normal |
| Renal function tests | BUN and creatinin were normal. Urinalysis was normal except for microscopic hematuria and 1+ protein |
| Liver function tests | ALT, AST, alkaline phosphatase and GGT were normal. LDH mildly increased; total protein and albumin were decreased significantly |
| Serologic tests | ASO titer,Wright,2ME,Coomb’s Wright, and Widal tests all were negative |
Blood, urine, and stool cultures were negative. On day four of admission, she developed a temporary diplopia and dizziness which improved after 48 hours without any specific intervention. A cardiac consult was requested in which there was just mild mitral regurgitation but no evidence of pericardial effusion, coronary involvement or vegetation were noted. PPD test was non-reactive; chest X-ray was normal. On the 6th day of admission, she was still febrile, anorexic and pale with severe limb pain and inability to walking; It appeared that collagen vascular diseases were worked up while results were negative for ANA, anti-ds DNA, anti-GBM antibody, and circulatory immune complex (CIC). Complement levels were also normal. Ophthalmologic consult findings were not compatible with an active rheumatologic disease. A microscopic agglutination test for Leptospira spp. was requested which revealed to be positive. Hence, a PCR test on peripheral blood and stool samples for leptospira was performed and both were positive. Materials and methods for PCR were as follows:
Master Mix Taq DNA poly: 1U
dNTP (dCTP, dGTP, dTTP): 250µm, TrisHCl (pH= 10mM, KCl: 30mM, MgCl2: 1.5mM, tEmplate DNA: 20ng, primer F, R: 10 pmol, H2O up to 20λ)
Thermal cycler ABI 2720
2.1. Test Procedure
For each patient 0.25µL of primer F and 0.25 µL of primer R and 9.5 µL of H2O and 10 µL of extracted DNA is added to lyophilized tube with usual temperature profile tubes is placed on the thermal cycler. Electrophoresis of the PCR product on 2% agarose v gel containing Rima SI g ht in TBE buffer at voltage 80-100 v for 30 minute. The gel is evaluated with UV transluminator. For each test one positive control; one NTC and one DNA marker are evaluated simultaneously on the same gel. The gel is shown in Figure 1.
Results of PCR
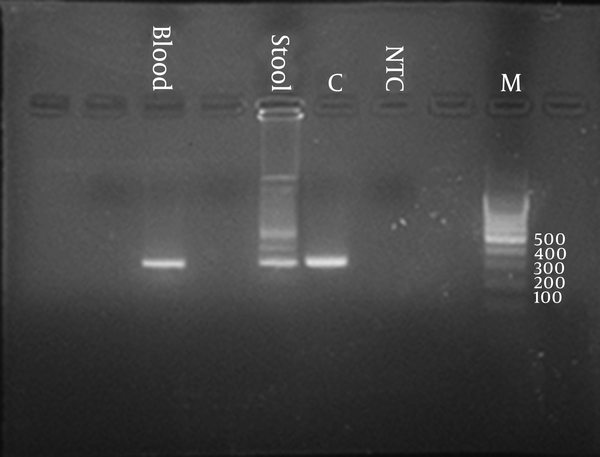
Doxycycline was started after which fever subsides gradually. Her complaints all resolved after 10 days. Her CBC and platelet counts were normal and sedimentation rate dropped to 13 mm/1th hour after 3 weeks of treatment. After 1 year follow-up, she was quite normal in terms of clinical and laboratory findings.
3. Conclusions
Leptospirosis is a zoonotic disease of global importance, caused by spirochetes of the genus Leptospira. Based on antigenic relatedness, human pathogenic Leptospirainterrogans strains have been differentiated into > 200 serovars, classified into 24 serogroups (1). A variety of wild and domestic animals form the natural reservoir for pathogenic leptospires. Transmission to humans results from exposure to the urine of infected animals either by direct contact or through contaminated soil or water (2). Recent surveillance data indicate an increase in disease incidence in some countries; for example in Germany the incidence has increased to 0.06 per 100,000 from 1998 to 2003 (2).
Leptospirosis is present in almost all world areas except poles; China, India and Brazil are among the most contaminated areas (3). Although most cases in Iran have been reported from northern parts of the country with 192 cases from Gilan province in 1997, but serologic investigation of cattle in some central provinces like Arak and Qom has shown considerable positivity indicative of spreading status of the disease in the country (3). Southwestern part of Iran is also probably contaminated as in a study in Ahvaz in 2003, 15% of samples from sheep were positive for Leptospiraspp; some of them had positivity for more than one species (4). Some serovars like pomona followed by canicola ,icterohaemorrhagiae, grippotyphosawere more prevalent in this study; but icterohaemorrhagiae was the most common serovar from northern part of the country (3). Although serologic investigation of human Leptospirosis in tribal areas of central west provinces has also shown strong positivity (48.5%) at a minimum titer of 1/100 especially to serovarhardjo, Dezful has not been recognized as an enzootic focus in Iran. So this case shows that other parts of country might be contaminated now and this diagnosis should be in mind for every case with compatible clinical picture.
Clinical presentation of Leptospirosis is quite misleading and it is simply overlooked even in countries with high grade of endemicity. In a study in Turkey, The most frequent initial symptoms and findings were fever, fatigue, headache, nausea and vomiting and increased muscle sensitivity which all can be misdiagnosed by a viral infection (5). Kawasaki disease in children and other collagen vascular diseases might also be confused with Leptospirosis as in our case there was severe body pain and conjunctival involvement. In the absence of hepatic and renal involvement and with high rate of erythrocyte sedimentation rate this case could be potentially diagnosed as a nonspecific vasculitis and become a candidate for taking IVIG or corticosteroids, probably both with no result; these treatments might increase the diagnostic problem as checking antibodies could be potentially affected by former and a nonspecific anti-inflammatory action of corticosteroids may affect the acute phase reactant indicators. Therefore, it is very important to diagnose cases with Leptospirosis properly. The employment of Warthin-Starry silver staining, polymerase chain reaction, and immunofluorescent, immunohistochemical methods and microscopic agglutination test can be useful for diagnosis, each with its benefits and limitations (6). In Iran, the microscopic agglutination test is being used for years with good results, but for more than one decade the usage of different types of primers for PCR methods have been encouraging (6-8). Early diagnosis with PCR methods can potentially permit the physicians to start treatment on time as for most serologic tests including microscopic agglutination test it may need more than 10 days to become diagnostic and there are some evidence that delayed treatment might not be effective for patients (6). Presentation of this case with nonspecific severe constitutional signs and symptoms and compatible sex and age for collagen vascular diseases especially with high sedimentation rate could be misleading, especially when she comes from non-endemic area for leptospirosis, so given the spreading areas of such a zoonotic disease in Iran it is very important to consider leptospirosis as an important differential diagnosis of vasculitis syndromes even in patients from apparently non-infected areas.
Acknowledgements
References
-
1.
Vinetz JM. Leptospirosis. Curr Opin Infect Dis. 2001;14(5):527-38. [PubMed ID: 11964872].
-
2.
Jansen A, Schöneberg I, Frank C, Alpers K, Schneider T, Stark K. Leptospirosis in Germany, 1962–2003. Emerging infectious diseases. 2005;11(7):1048-54.
-
3.
Nateghian AR, Joafshani MA, Zoghi E, Simani S, Mardani M, Mohebali M, et al. Common zoonosis in Iran. Tehran: Ettelaat publication. Ministry of Health and Medical Education; 1995.
-
4.
Haji Hajikolaei MR, Ghorbanpour M, Gharibi D, Abdollapour GR. Serologic study on leptospiral infection in sheep in Ahvaz, southwestern Iran. Iranian J Vet Res. 2007;8:333-6.
-
5.
Turhan V, Polat E, Murat Atasoyu E, Ozmen N, Kucukardali Y, Cavuslu S. Leptospirosis in Istanbul, Turkey: a wide spectrum in clinical course and complications. Scand J Infect Dis. 2006;38(10):845-52. [PubMed ID: 17008227]. https://doi.org/10.1080/00365540600681542.
-
6.
David HD, Rosenberg MB. Leptospira. Nelson textbook of Pediatrics. 19 ed. Philadelphia: Saunders; 2011.
-
7.
Wangroongsarb P, Yaseang S, Petkanjanapong W, Naigowit P, Hagiwara T, Kawabata H, et al. Applicability of Polymerase Chain Reaction to Diagnosis of Leptospirosis. J Trop Med Parasitol. 2005;28:43-7.
-
8.
Brown PD, Gravekamp C, Carrington DG, van de Kemp H, Hartskeerl RA, Edwards CN, et al. Evaluation of the polymerase chain reaction for early diagnosis of leptospirosis. J Med Microbiol. 1995;43(2):110-4. [PubMed ID: 7629850].