Abstract
Keywords
1. Introduction
We would like to share our experience with an eleven-year-old male, a case of acquired aplastic anemia as a complication of disseminated tuberculosis with acute onset, rapid downhill course, and refractory to both anti-tubercular as well as immunosuppressive drugs.
2. Short Communication
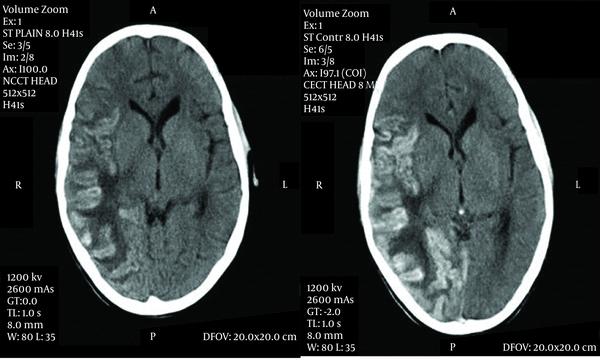
Tuberculosis is known to cause wide variety of systemic complications when it disseminates in the body. Tuberculosis, suppressing all three cell lines which causes pancytopenia, leading to aplastic anemia has been reported by very few case reports, either as the result of disseminated tuberculosis or as an adverse effect of anti-tubercular medicines (1-3). An eleven-year-old male, weighing 27 kg with poor nutritional status presented to our pediatric intensive care unit with history of fever for one month, headache and vomiting for 15 days and weakness of right half of his body with right-sided deviation of the angle of his mouth for 3 days. He also noticed bluish spots on the chest and trunk for one day. He lived in an overcrowded house with illiterate parents and belonged to a family with low socioeconomic status, but was immunized and had BCG scar with a history of contact with an active tuberculosis patient in his family. He did not have similar complaints in the past or any hospitalization or medication. On examination, the child was afebrile and normotensive, his heart rate was 110 beats per minute, and respiratory rate was 27/min. He was severely pale, and petechiae were observed over the trunk accompanied by subconjunctival hemorrhage. He was drowsy with altered sensorium. Neurological examination revealed meningoencephelitis with right sided hemiparesis with left sided facial palsy. The examination of other systems was within normal limits. Cerebrospinal fluid analysis revealed 432 mg/dL protein, 43 mg/dL glucose, 110 meq/L chloride with 160 cells out of which 75% was lymphocytes and 25% was polymorphs. Gram staining of the CSF was unremarkable and culture was negative. However, CSF PCR was positive for mycobacteria tuberculosis. There were few signs of raised intracranial pressure without active seizure. He was given supportive treatment with intravenous fluids, mannitol, and dexamethasone. When he was stabilized on the second day, antitubercular therapy and nasogastric tube feeding were initiated. Brain CT showed gyral swelling with patchy hemorrhage involving fronto-parieto-temporo-occipital lobe (meningo-encephelitis) with grade 3 perifocal edema leading to mild leftward midline shift (Figure. 1).
Axial Contrast-Enhanced CT Scan Images of The Brain Demonstrating Patchy Gyral Hemorrhage With Leptomeningeal Enhancement Involving the Right Temporo-Parieto-Occipital Region With Extensive Edema and Midline Shift
Hematological deterioration continued as pancytopenia was persistent on peripheral blood smear examination. Peripheral smear did not show any abnormal cells, hemoparasites, or evidence of hemolysis. Bone marrow aspiration confirmed hypoplastic marrow with epitheloid cell granuloma and focal necrosis consistent with tuberculosis. There were no features suggestive of hemophagocytic syndrome in bone marrow smear examination. The bleeding time was 14 minutes. The clotting, prothrornbin and partial thromboplastin time were normal. The urine and stool examination did not show any abnormality. Urine and blood cultures did not grow any organisms. The direct and indirect Coomb's tests were negative. Blood Widal, HIV ELISA and VDRL were non-reactive. We added anabolic steroids and later cyclosporine for which there was no response. There was no possibility to transfer the patient to another center with facilities for bone marrow transplantation. In spite of two platelet-rich plasma transfusions, the child started bleeding due to severe thrombocytopenia, developed hypovolumic shock and died as the result of massive uncontrollable upper gastrointestinal and pulmonary hemorrhages after 19 days of hospital stay. Autopsy could not be performed.
Literature shows the occurrence of pancytopenia in disseminated tuberculosis is attributed to hypersplenism, histiocytic hyperplasia and indiscriminate phagocytosis of blood cells by histiocytes in the bone marrow, maturational arrest, or infiltration of the bone marrow by caseating or noncaseating tubercular granulomas. Tubercular granulomas may cause pancytopenia by replacement of marrow cells or suppression through release of interferon and lymphotoxin. However, despite presence of tuberculous granulomas in a high proportion of patients with disseminated tuberculosis, pancytopenia is uncommon (1). Pathophysiology of acquired aplastic anemia in tuberculosis is found to be immune-mediated based on observation of recovery after immunosuppressant, but the present case was resistant to this treatment (4).
3. Conclusions
There are several unusual features of interest in this case. Amidst many reports which have not shown association between tuberculosis and aplastic anemia, our case brings back the old view to support the causation of aplastic anemia in tuberculosis (5). Aplasia of the bone marrow was caused by an overwhelming toxemia. Surprisingly no system was able to analyze the effect of the condition on bone marrow, disregarding the neurological aspects. In spite of limiting to two foci, severity of the bone marrow disease was rapidly progressive and did not respond to supportive treatment.
Acknowledgements
References
-
1.
Yadav TP, Mishra S, Sachdeva KJ, Gupta VK, Siddhu KK, Bakshi G, et al. Pancytopenia in disseminated tuberculosis. Indian Pediatr. 1996;33(7):597-9. [PubMed ID: 8979574].
-
2.
Avasthi R, Mohanty D, Chaudhary SC, Mishra K. Disseminated tuberculosis: interesting hematological observations. J Assoc Physicians India. 2010;58:243-4. [PubMed ID: 21046880].
-
3.
Kernohan EJ. Aplastic anaemia complicating miliary tuberculosis. Br Med J. 1950;2(4675):399-400. [PubMed ID: 15434400].
-
4.
Young NS. Pathophysiologyic mechanism in acquired aplastic anemia. Heamatol. 2006:72-7.
-
5.
Hsu HC, Lee YM, Su WJ, Huang CY, Yang CF, Ho CK, et al. Bone marrow samples from patients with aplastic anemia are not infected with parvovirus B19 and Mycobacterium tuberculosis. Am J Clin Pathol. 2002;117(1):36-40. [PubMed ID: 11789728]. https://doi.org/10.1309/B361-LA0V-UX0X-GJPQ.