Abstract
Background:
Ventilator-associated pneumonia (VAP) is a type of nosocomial pneumonia which develops more than 48 hours after endotracheal intubation. Early recognition and treatment of VAP is important, since timely and appropriate management can be lifesaving.Objectives:
This study aimed to determine the antimicrobial susceptibility pattern of microorganisms causing VAP in the intensive care units (ICU) of two university associated hospitals in the province of Mazandaran in Iran from 2008 to 2010.Materials and Methods:
This study was performed on VAP patients diagnosed with the clinical pulmonary infection score (CPIS) in ICU’s of two university hospitals. For each patient suspected of VAP, quantitative culture of endotracheal aspiration (QEA) was performed and minimum inhibitory concentration (MIC) was determined by a micro dilution test. Data was analyzed by the SPSS 17 software and a P < 0.05 was considered to be statistically significant.Results:
In this study, the type and the frequency of the microbial agents causing VAP was as follows: coagulate negative staphylococci (23.3%), Escherichia coli (E. coli) (21.7%), Staphylococcus aureus (S. aureus) (18.3%), Pseudomonas aeruginosa (P. aeroginosa) (18.3%), Enterobacter spp (11.7%). and Klebsiella pneumoniae (K. pneumonia) (6.7%). 35.71% of coagulate negative staphylococci were sensitive to vancomycin. All of the isolated E. coli was resistant to ceftazidime, but 50% sensitive to gentamicin and meropenem. 54.54% of isolated S. aureus were resistant to vancomycin. All of the isolated P. aeroginosa cases were sensitive to imipenem while 50% were resistant to ceftazidime.Conclusions:
In patients with VAP, carbapenems had good activity against P. aeroginosa. Increasing resistance of S. aureus to vancomycin requires more attention and further studies.Keywords
1. Background
Ventilator-associated pneumonia (VAP) refers to pneumonia that develops at least 48 hours after the initiation of mechanical ventilation (1, 2). It is most likely caused by aerodigestive tract colonization, followed by aspiration of contaminated secretions into the lower airways. Hence, factors that increase the risk of colonization and aspiration increase the risk of VAP, while preventing these events can significantly reduce the risk of its occurrence (3-8). Diagnostic dilemmas intensify in practice settings where lung biopsies are seldom obtained (9-11). Most clinical assessment tools and guidelines have incorporated a mixture of symptoms, signs, radiographic findings, and culture results in their diagnostic approach to VAP. The Clinical Pulmonary Infection Score (CPIS) represents one such approach.
A number of studies from the past two decades have identified cumulative incidence rates of 0.5 to 1.0% in U.S. hospitals. Extrapolations of these figures to hospital admission data suggest that 250,000 to 300,000 cases of nosocomial pneumonia occur annually. Hospital-wide surveillance studies have average incidence rates of 0.8 cases per 1000 patient-care days (3, 7). The results of microbiologic studies allow us to reduce the scope of antimicrobial therapy to target only the isolated pathogen(s). In two studies, negative PSB and BAL cultures allowed discontinuation of antimicrobial therapy (12, 13).
Antimicrobial resistance has emerged as an important determinant of outcome for patients in the intensive care unit. This is largely due to the administration of inadequate antimicrobial treatment, which is most often related to bacterial antibiotic resistance. Intensive care units are unique because they house seriously ill patients in confined environments where antibiotic use is extremely common. They have been focal points for the emergence and spread of antibiotic-resistant pathogens. Effective strategies for the prevention of antimicrobial resistance in ICU settings have focused on limiting the unnecessary use of antibiotics and increasing compliance with infection control practices. Clinicians caring for critically ill patients should consider antimicrobial resistance as part of their routine treatment plans. Careful and focused attention to this problem at the ICU and using a multidisciplinary approach, will have the greatest likelihood of limiting the development and dissemination of antibiotic-resistant infections (14).
2. Objectives
This study was designed to determine the antimicrobial susceptibility pattern of microorganisms causing VAP by the minimal inhibitory concentrations minimal inhibitory concentrations (MIC) determination method.
3. Materials and Methods
This study was performed on patients suspected of VAP in ICUs of two university associated hospitals in the province of Mazandaran in Iran from 2008 to 2010. Cases that had a CPIS score of < 6 were excluded from this study. After calculation of the CPIS score, cases that were suspected of VAP were further investigated. The clinical pulmonary infection score, used in some ICUs, gives points for clinical, radiographic, physiologic, and microbiologic data for a single numerical result. A clinical pulmonary infection score of more than 6 correlates well with the presence of clinical pneumonia (Table 1) (15).
| Criterion | Value | Points |
|---|---|---|
| Temperature, °C | ≥ 36.5 and ≤ 38.4 | 0 |
| ≥ 38.5 and ≤ 38.9 | 1 | |
| ≤ 36.0 and ≥ 39.0 | 2 | |
| Blood leukocyte, μL | ≥ 4000 and ≤ 11,000 | 0 |
| < 4000 or > 11,000 | 1 | |
| ≥ 500 band forms | 1 | |
| Tracheal secretions | Absence of tracheal secretions | 0 |
| Presence of nonpurulent tracheal secretions | 1 | |
| Presence of purulent tracheal secretions | 2 | |
| Oxygenation (PaO2/FiO2)a, mm Hg | > 240 or ARDSb | 0 |
| ≤ 240 and no evidence of ARDS | 2 | |
| Pulmonary radiography | No infiltrate | 0 |
| Diffuse or patchy infiltrate | 1 | |
| Localized infiltrate | 2 | |
| Progression of pulmonary infiltrate | No radiographic progression | 0 |
| Radiographic progression (after CHFband ARDS excluded) | 2 | |
| Culture and gram stain of tracheal aspirate | No pathogenic bacteria cultured | 0 |
| Pathogenic bacteria cultured | 1 | |
| Some pathogenic bacteria seen on Gram stain | 1 |
The microorganisms in these cases were isolated and their MIC was determined by the micro dilution test. This was achieved by obtaining the pulmonary secretion of these cases via intubation and endotracheal aspiration. Subsequently, these collected specimens were sent to our clinical microbiology laboratory (from July 2008 to March 2010). Specimens submitted to the laboratory were cultured on Mueller-Hinton Agar, blood agar and Todd-Hewitt broth medium. Quantitative positive culture was > 100000 cfu/mL. Microorganism isolates were identified by conventional laboratory approaches, including Gram stain and colony morphology. MICs for cloxacillin, vancomycin, ceftriaxone, ceftazidime, amikacin, gentamicin, imipenem and meropenem were determined by broth micro dilution as recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and MICs were read manually after 24 hours of incubation (17). The MIC breakpoints that were used are based on the established criteria by the European Committee on Antimicrobial Susceptibility Testing version 1.3, fifth of January 2011. Data collection and analysis was done using the SPSS 17, and differences were considered to be significant for P < 0.05.
4. Results
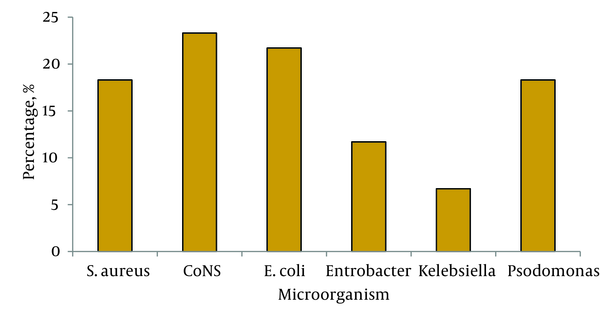
Our study included 60 cases of VAP based on their CPIS scores. In this population, 68.3% (41 cases) had underlying diseases including various forms of malignancy (34%), heart disease (14.7%), DM (12.2%) and trauma (39.1%). Of the 60 isolated strains, the frequency of different microorganisms were as follows: coagulase negative staphylococci 23.3% (14 cases), Escherichia coli 21.7% (13 cases), Staphylococcus aureus 18.3% (11 cases), Pseudomonas aeroginosa 18.3% (11 cases), Enterobacter spp. accounted for 11.7% (7 cases) and finally Klebsiella pneumonia 6.7% (4 cases) (Figure 1).
Percentage of Microorganisms Isolated from 60 Cases of VAP Patients
Of the isolated strains of coagulase negative staphylococci, 35.71% were sensitive to vancomycin and 64.28% were fully resistant. About eighty five percent (85.71%) were resistant against cloxacilline. About thirty six percent (36.36%) of the isolated strains of Staphylococcus aureus were cloxacillin sensitive strains and the remainders were resistant against (methicillin resistant Staphylococcus aurous (MRSA)). About forty five percent (45.45%) of cases were sensitive to vancomycin and for 6 cases (54.54%) MIC was greater than 2 mcg/mL (resistant cases) (Table 2, 3).
Antimicrobial Susceptibility Pattern of Gram Positive Bacteria
| Antibiotic | Sensitivity | Coagulase-Negative Staphylococci, % | Staphylococcus aureus, % |
|---|---|---|---|
| Cloxacilline | Sa | 36.36 | 14.28 |
| Ia | 9.09 | 0.0 | |
| Ra | 54.54 | 85.71 | |
| Gentamycin | S | 9.09 | 0.0 |
| I | 0.0 | 0.0 | |
| R | 90.90 | 100 | |
| Amikacin | S | 9.09 | 57.14 |
| I | 0.0 | 0.0 | |
| R | 90.90 | 42.85 | |
| Ceftriaxone | S | 0.0 | 14.28 |
| I | 18.18 | 0.0 | |
| R | 81.81 | 85.71 | |
| Ceftazidime | S | 18.18 | 7.14 |
| I | 45.45 | 7.14 | |
| R | 36.36 | 85.71 | |
| Imipeneme | S | 72.72 | 42.85 |
| I | 27.27 | 14.28 | |
| R | 0.0 | 42.85 | |
| Meropenem | S | 81.81 | 71.42 |
| I | 18.18 | 0.0 | |
| R | 0.0 | 28.57 | |
| Vancomycin | S | 45.45 | 35.71 |
| I | 0.0 | 0.0 | |
| R | 45.5 | 64.28 |
Antimicrobial Susceptibility Pattern of Gram Negative Bacteria
| Antibiotic | Sensitivity | Pseudomonas aeruginosa, % | Klebsiella pneumoniae, % | Escherichia coli, % | Enterobacter, % |
|---|---|---|---|---|---|
| Ceftazidime | S a | 45.45 | 0.0 | 0.0 | 0.0 |
| I a | 0.0 | 0.0 | 0.0 | 0.0 | |
| R a | 54.54 | 100 | 100 | 100 | |
| Ceftriaxone | S | NAa | 0.0 | 7.69 | 0.0 |
| I | NA | 0.0 | 15.38 | 0.0 | |
| R | NA | 100 | 76.92 | 100 | |
| Gentamycin | S | 54.54 | 50 | 46.15 | 42.85 |
| I | 0.0 | 50 | 7.69 | 28.57 | |
| R | 45.45 | 0.0 | 46.15 | 28.57 | |
| Amikacin | S | 54.54 | 100 | 23.07 | 71.42 |
| I | 0.0 | 0.0 | 7.69 | 0.0 | |
| R | 45.45 | 0.0 | 69.23 | 28.57 | |
| Imipeneme | S | 100 | 75 | 23.07 | 71.42 |
| I | 0.0 | 25 | 30.76 | 28.57 | |
| R | 0.0 | 0.0 | 46.15 | 0.0 | |
| Meropenem | S | 90.90 | 75 | 46.15 | 85.71 |
| I | 0.0 | 25 | 0.0 | 14.28 | |
| R | 9.09 | 0.0 | 53.84 | 0.0 |
5. Discussion
The incidence of VAP in patients receiving mechanical ventilation is estimated to be approximately 22.8% (18). The cost of VAP is estimated to be $40000 per hospital admission per patient with VAP and its estimated annual cost being approximately $1.2 billion dollars in the USA (19). Antimicrobial resistance is a threat to public health, worldwide and is associated with higher mortality and morbidity rates. Despite extensive knowledge about this issue, drug resistance has continued to emerge, especially in ICUs. In our study, the type and frequency of microbial agents causing VAP was as follows: coagulase negative staphylococci (23.3%), E coli (21.7%), S. aureus (18.3%), P. aeroginosa (18.3%), Enterobacter spp (11.7%). and K. pneumonia (6.7%). In a study by Heyland et al. the infecting flora in patients with VAP included methicillin-sensitive Staphylococcus aurous (MSSA) (9%), MRSA (18%), P. aeroginosa (18%), Stenotrophomonas maltophilia (7%), Acinetobacter spp (8%), and other spp (9%). These findings are supported by a prospective, multicenter, observational study of 398 ICU patients with suspected VAP (20). In this study, there was a similar distribution of pathogens-MRSA (14.8%), P. aeruginosa (14.3%), and other Staphylococcus species (8.8%) (21). The frequency of bacterial agents causing VAP varies in different studies. In some studies the most common pathogen was S. aureus while in others it was P. aeroginosa with low frequency for coagulase negative staphylococci. In a number of recent studies, the most common pathogens identified on culture of patients with VAP were gram-negative bacteria, S. aureus, and H. influenzae (18, 22).
Lambiase and colleagues performed a microbiological analysis of 29 suspected VAPs patients. In their study, for 15 cases (51.7%) the responsible microorganism was P. aeroginosa, while in the other 14 cases (48%) a number of different bacteria were isolated, including Enterobacter spp (17.24%), Acinetobacter baumannii (17.24%), Staphylococcus aureus (3.44%), Klebsiella pneumoniae (3.44%), Escherichia coli (3.44%), and Haemophilus influenzae (3.44%) (23). Our study indicated a high resistant rate especially among the gram-positive cocci. Coagulase negative staphylococci showed resistance rates of 85.71%, 64.28% and 42.85% to cloxacillin, vancomycin and amikacin, respectively. S. aureus showed resistance rates of 54.54%, 54.54%, 90.90% and 90.90% to cloxacillin, vancomycin, gentamicin and amikacin, respectively. In a study by Wang and colleagues a total of 6,003 S. aureus isolates were analyzed from 2000 to 2004. No vancomycin-resistant S. aureus isolates were detected. One MRSA isolate had a vancomycin MIC of 8 mcg/mL and was confirmed as a vancomycin-intermediate S. aureus (24) while in our study for 6 cases (54%) MIC was greater than 2 mcg/mL (full resistant cases). In the study by Japoni et al. it was showen that three antibiotics including linezolid, vancomycin, and quinupristin/dalfopristin showed high coverage in gram-positive bacteria. The gram-negative bacteria in that study were highly sensitive to colistin, meropenem, and imipenem (25). In a study by Zervos et al. they found that mortality from all causes at day 28 was 32.3%. The majority of MRSA isolates had a vancomycin MIC ≥ 1.5 mcg/mL (115/158, 72.8%). Propensity score analysis demonstrated an increase in 28-day mortality as vancomycin MIC increased from 0.75 to 3 mcg/mL (P ≤ .001) (26).
P. aeroginosa isolated in our study were sensitive to carbapenems (100% to imipenem and 90% to meropenem) and 50% were resistant to ceftazidime. This sensitivity differs from the study of de carvalho in Brazil that isolated P. aeruginosa with resistance above 70.0% to third generation cephalosporins and imipenem (27). While a surveillance center in the USA as part of an Intensive Care Antimicrobial Resistance Epidemiology (ICARE) program reported that resistance rates of P. aeruginosa, to fluoroquinolons, imipenem and third generation cephalosporins were 35.0%, 19.0% to and 14.0%, respectively (28). Also, a surveillance program center in Germany (SARI) reported a resistance of 18.0% to fluoroquinolons, 25.4% to imipenem and 15.3% to third generation cephalosporins (29). In a laboratory detection study of imipenem or meropenem resistance in gram-negative organisms, isolates of Pseudomonas aeruginosa had MICs that were at or near the carbapenem intermediate (8 µg/mL) and resistant (> 16 µg/mL) breakpoints (30). In April 2006, in a tertiary care center in Medellin, Colombia, three imipenem-resistant isolates of P. aeruginosa (MIC ≥ 256 µg/mL) were recovered. Two of the isolates were from patients with ventilator-associated pneumonia (31). In our study, we found increased resistance of E. coli to 3rd generation cephalosporins, aminoglycosides and carbapenems as well as high-level resistance to ceftazidime (50 mcg/mL). The higher rate of third generation cephalosporins-resistant E. coli in our study (> 90%) is significantly different from the rates reported by De Carvalho in Brazil (18.7%) (26) and the Sentry (surveillance program in Brazil) program (4.4%) (32).
In a study by Mendes and colleagues, they found that E. coli was fully susceptible to imipenem and meropenem (33). In addition, carbapenem resistance among Enterobacteriaceae was still rare in that region (32). This finding also significantly differs with our results. The European Antimicrobial Resistance Surveillance System described resistance against 3rd generation cephalosporin (3GC) in E. coli as the most dynamic expansion of multidrug-resistant pathogens in the entire region (34). Although in 2008, less than one-half of European countries (14 of 33) reported their resistance levels against 3GC to be under 5%. Since 2004, the proportion of 3GC resistance has increased in 19 European countries. In general, a large percentage of ESBL-producing pathogens are now being imported into hospitals and ICUs (35-37). Meyer and colleagues have reported that the rate of Escherichia coli resistance to third generation cephalosporins has significantly increased between 2001 and 2008 (1.2% and 19.7% respectively with P < 0.001). The sharp increase in 3GC-resistant E. coli started in 2006 and affected almost all ICUs (38). Klebsiella enterobacter group isolated in our study presented high level resistance to third generation cephalosporins and almost full sensitivity to carbapenems. However, we could use imipenem/meropenem for the treatment of these patients with VAP. The number of cases belonging to this group was low in our sample and we could not compare them to other studies for analysis. VAP causing microorganisms in our region have become increasingly resistant to antibiotics which are commonly used in empirical treatment of this disease in our local ICUs. This further illustrates the need for more antimicrobial susceptibility tests and surveillance programs in our critical care units.
Acknowledgements
References
-
1.
Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care. 2005;50(6):714-21. [PubMed ID: 15913464].
-
2.
Rello J, Diaz E. Pneumonia in the intensive care unit. Crit Care Med. 2003;31(10):2544-51. [PubMed ID: 14530765]. https://doi.org/10.1097/01.CCM.0000089928.84326.D2.
-
3.
Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416. [PubMed ID: 15699079]. https://doi.org/10.1164/rccm.200405-644ST.
-
4.
Coffin SE, Klompas M, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S31-40. [PubMed ID: 18840087]. https://doi.org/10.1086/591062.
-
5.
Craven DE. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest. 2006;130(1):251-60. [PubMed ID: 16840410]. https://doi.org/10.1378/chest.130.1.251.
-
6.
Dodek P, Keenan S, Cook D, Heyland D, Jacka M, Hand L, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141(4):305-13. [PubMed ID: 15313747].
-
7.
Hess DR, Kallstrom TJ, Mottram CD, Myers TR, Sorenson HM, Vines DL, et al. Care of the ventilator circuit and its relation to ventilator-associated pneumonia. Respir Care. 2003;48(9):869-79. [PubMed ID: 14513820].
-
8.
Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R, CDC, Healthcare Infection Control Practices Advisory Committee, et al. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1-36. [PubMed ID: 15048056].
-
9.
Craven DE, Steger KA. Hospital-acquired pneumonia: perspectives for the healthcare epidemiologist. Infect Control Hosp Epidemiol. 1997;18(11):783-95. [PubMed ID: 9397379].
-
10.
Mayhall CG. Nosocomial pneumonia: diagnosis and prevention. Infect dis clin N Am. 1997;11(2):427-57.
-
11.
Bonten MJM, Bergmans DCJJ. Nosocomial pneumonia. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999. 211–38 p.
-
12.
Wu CL, Yang DIe, Wang NY, Kuo HT, Chen PZ. Quantitative culture of endotracheal aspirates in the diagnosis of ventilator-associated pneumonia in patients with treatment failure. Chest. 2002;122(2):662-8. [PubMed ID: 12171848].
-
13.
Heyland DK, Cook DJ, Marshall J, Heule M, Guslits B, Lang J, et al. The clinical utility of invasive diagnostic techniques in the setting of ventilator-associated pneumonia. Canadian Critical Care Trials Group. Chest. 1999;115(4):1076-84. [PubMed ID: 10208211].
-
14.
Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Internal Med. 2001;134(4):298-314.
-
15.
Strausbaugh LJ. Nosocomial respiratory infections. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 6th ed. London: Churchill Livingstone; 2005. 3363 p.
-
16.
Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505-11. [PubMed ID: 10934078]. https://doi.org/10.1164/ajrccm.162.2.9909095.
-
17.
EUCAST Steering Committee Meeting. Munich, Germany; 3-4 April 2007. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/3Research_Projects/EUCAST/Meetings/steering_meetings/Steering%20committee%20meeting%20070403%20summary%20ratified%20r.pdf.
-
18.
Augustyn B. Ventilator-associated pneumonia: risk factors and prevention. Crit Care Nurse. 2007;27(4):32-9. quiz 40. [PubMed ID: 17671243].
-
19.
van Nieuwenhoven CA, Buskens E, Bergmans DC, van Tiel FH, Ramsay G, Bonten MJ. Oral decontamination is cost-saving in the prevention of ventilator-associated pneumonia in intensive care units. Crit Care Med. 2004;32(1):126-30. [PubMed ID: 14707570]. https://doi.org/10.1097/01.CCM.0000104111.61317.4B.
-
20.
Fagon JY, Chastre J, Wolff M, Gervais C, Parer-Aubas S, Stephan F, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132(8):621-30. [PubMed ID: 10766680].
-
21.
Kollef MH, Morrow LE, Niederman MS, Leeper KV, Anzueto A, Benz-Scott L, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest. 2006;129(5):1210-8. [PubMed ID: 16685011]. https://doi.org/10.1378/chest.129.5.1210.
-
22.
Barclay L, Vega C. Ventilator-associated Pneumonia linked to worse outcomes in critically ill children. Pediatrics. 2009;;123:1108–15.
-
23.
Lambiase A, Rossano F, Piazza O, Del Pezzo M, Catania MR, Tufano R. Typing of Pseudomonas aeruginosa isolated from patients with VAP in an intensive care unit. New Microbiol. 2009;32(3):277-83. [PubMed ID: 19845110].
-
24.
Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44(11):3883-6. [PubMed ID: 16957043]. https://doi.org/10.1128/JCM.01388-06.
-
25.
Japoni A, Vazin A, Davarpanah MA, Afkhami Ardakani M, Alborzi A, Japoni S, et al. Ventilator-associated pneumonia in Iranian intensive care units. J Infect Dev Ctries. 2011;5(4):286-93. [PubMed ID: 21537070].
-
26.
Haque NZ, Zuniga LC, Peyrani P, Reyes K, Lamerato L, Moore CL, et al. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138(6):1356-62. [PubMed ID: 20558550]. https://doi.org/10.1378/chest.09-2453.
-
27.
de Carvalho RH, Gontijo Filho PP. Epidemiologically relevant antimicrobial resistance phenotypes in pathogens isolated from critically ill patients in a Brazilian Universitary Hospital. Braz J Microbiol. 2008;39(4):623-30. [PubMed ID: 24031278]. https://doi.org/10.1590/S1517-83822008000400005.
-
28.
National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470-85. [PubMed ID: 15573054]. https://doi.org/10.1016/S0196655304005425.
-
29.
Meyer E, Jonas D, Schwab F, Rueden H, Gastmeier P, Daschner FD. Design of a surveillance system of antibiotic use and bacterial resistance in German intensive care units (SARI). Infection. 2003;31(4):208-15. [PubMed ID: 14562943].
-
30.
[updated November 24, 2010]. Laboratory detection of imipenem or meropenem resistance in gram-negative organisms. Available from: http://www.cdc.gov/HAI/settings/lab/lab_imipenem.html.
-
31.
Villegas MV, Lolans K, Correa A, Kattan JN, Lopez JA, Quinn JP, et al. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob Agents Chemother. 2007;51(4):1553-5. [PubMed ID: 17261621]. https://doi.org/10.1128/AAC.01405-06.
-
32.
Sader HS, Jones RN, Gales AC, Silva JB, Pignatari AC. SENTRY antimicrobial surveillance program report: Latin American and Brazilian results for 1997 through 2001. Braz J Infect Dis. 2004;8(1):25-79. [PubMed ID: 15286878].
-
33.
Mendes C, Oplustil C, Sakagami E, Turner P, Kiffer C. Antimicrobial susceptibility in intensive care units: MYSTIC program Brazil 2002. Braz J Infect Dis. 2005;9(1):44-51. [PubMed ID: 15947846].
-
34.
European Antimicrobial Resistance Surveillance System annual report. 2008. Available from: http://www.rivm.nl/earss/Images/EARSS%202008_final_tcm61-65020.pdf.
-
35.
Harris AD, McGregor JC, Johnson JA, Strauss SM, Moore AC, Standiford HC, et al. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis. 2007;13(8):1144-9. [PubMed ID: 17953083]. https://doi.org/10.3201/eid1308.070071.
-
36.
Meyer E, Serr A, Schneider C, Utzolino S, Kern WV, Scholz R, et al. Should we screen patients for extended-spectrum beta-lactamase-producing enterobacteriaceae in intensive care units? Infect Control Hosp Epidemiol. 2009;30(1):103-5. [PubMed ID: 19067603]. https://doi.org/10.1086/592702.
-
37.
Rodriguez-Bano J, Navarro MD, Romero L, Martinez-Martinez L, Muniain MA, Perea EJ, et al. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J Clin Microbiol. 2004;42(3):1089-94. [PubMed ID: 15004058].
-
38.
Meyer E, Schwab F, Schroeren-Boersch B, Gastmeier P. Dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Crit Care. 2010;14(3):R113. [PubMed ID: 20546564]. https://doi.org/10.1186/cc9062.