Abstract
Background:
Sepsis is one of the most important causes of morbidity and mortality in the intensive care units (ICUs). It is difficult to accurately differentiate sepsis from similar diseases rapidly. Therefore, it becomes critical to identify any biomarker with the ability of differentiation between sepsis and nonsepsis conditions. The urokinase plasminogen activator receptor has been implicated as an important factor in regulation of leukocyte adhesion and migration.Objectives:
In this study, we evaluated the value of soluble urokinase plasminogen activator receptor (suPAR), erythrocyte sedimentation (ESR), and C-reactive protein (CRP) serum levels in terms of their value for sepsis diagnosis in ICU patients.Patients and Methods:
We enrolled 107 ICU patients; 40 with sepsis, 43 with systemic inflammatory response syndrome, and 24 as control group. Serum soluble urokinase plasminogen activator receptor, ESR, white blood cell (WBC), and CRP levels were measured on the day of admission.Results:
The group with sepsis had higher suPAR, ESR, and CRP levels compared with the group with noninfectious systemic inflammatory response syndrome (SIRS) (P = 0.01, 0.00 and 0.00, respectively). CRP concentrations and ESR were higher in the sepsis group than in the non-SIRS group (P = 0.00 and 0.00, respectively). In a receiver-operating characteristic curve analysis, ESR, CRP and suPAR had an area under the curve larger than 0.65 (P = 0.00) in distinguishing between septic and noninfectious SIRS patients. CRP, ESR and suPAR had a sensitivity of 87%, 71% and 66% and a specificity of 59%, 76% and 74% respectively in diagnosing infection in SIRS.Conclusions:
The diagnostic values of CRP and ESR were better than suPAR and WBC count in patients with sepsis.Keywords
Plasminogen Systemic Inflammatory Response Syndrome C-Reactive Protein Sepsis
1. Background
Sepsis is one of the most important causes of morbidity and mortality in the intensive care units (ICUs). It also increases hospital stay and cost (1). Patients with fever, leukocytosis, increased heart rate and respiratory rate with suspected or proven infection can be diagnosed with sepsis. However, lack of sensitivity and specificity of these parameters makes it difficult to diagnose sepsis by these criteria alone. Blood culture has always been the golden standard for sepsis diagnosis. Treatment is normally delayed while waiting for culture result and it can be negative in many cases due to antibiotic administration or the absence of microbial invasion of the blood stream. It also can be delayed because of slow growing or fastidious organisms. Therefore, it is difficult to accurately differentiate sepsis from similar diseases rapidly. Besides, clinical studies have shown that early treatment can significantly improve the prognosis of sepsis. Therefore, it becomes critical to identify any biomarker with the ability of differentiation between sepsis and nonsepsis conditions (2, 3). A novel infectious disease biomarker is soluble urokinase-type plasminogen activator receptor (suPAR). The receptor (CD87), which is widely expressed on many different cell types including hematopoietic cells, has been implicated as an important factor in regulation of leukocyte adhesion and migration. Lipopolysaccharide, the toxic moiety of Gram-negative bacterial cell membrane, can enhance uPAR expression on monocytes (4). uPAR may be released from the cell surface by either cleavage of the glycolipid anchor by a phospholipase, or cleavage of the protein close to the anchor, thus forming a free soluble receptor (suPAR). suPAR is detected in low, but fairly constant concentrations in plasma of healthy, normal people (5). Concentrations of suPAR are increased in conditions that involved immune activation (6).
2. Objectives
In this study, we evaluated the value of suPAR, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) serum levels in terms of their value for sepsis diagnosis in ICU patients.
3. Materials and Methods
3.1. Patients
We studied 40 adults with sepsis, as defined by international guidelines (with or without identified pathogens), with no more than 24 hours of evolution, who were hospitalized in the ICU of Hazrat-e-Rasoul Akram Hospital, Tehran, Iran, from October 2009 to April 2010 (group A). Based on the American College of Chest Physicians/ Society of Critical Care Medicine (ACCP/SCCM) Sepsis Directory, patients exhibiting two or more of the following signs (infected with systemic inflammatory response syndrome (SIRS)) and suspected or proven infection were eligible for selection: 1- temperature of > 38˚C or < 36˚C, 2- pulse rate of > 90 beats/min, 3- respiratory rate of > 20 beats/min or hyperventilation with a partial pressure of arterial carbon dioxide (PaCO2) of < 32 mm Hg, or 4- white blood cell (WBC) count of > 12000 or < 4000 cells/µL, or > 10% immature cells (2). In addition, we analyzed 43 ICU patients with two or more SIRS criteria without any infection at the time of blood extraction as group B and 24 ICU patients without SIRS criteria as control group (group C). We acquired the acute physiology and chronic health evaluation (APACHE) IV score at the time of diagnosis, in addition to information on sex, age, temperature, principal diagnosis, vital signs, routine blood test and microbiological culture results. The study protocol was approved by the local ethics committee.
3.2. Laboratory Tests
Blood was obtained by clean venipuncture for culture and measurement of suPAR, CRP, ESR and WBC count. After centrifugation, the plasma was kept at -80˚C until assayed. Soluble uPAR was measured by quantitative enzyme-linked immunosorbent assay (ELISA) kit (USCN Life Science Inc. USA). CRP was measured by semi-quantitative latex agglutination method (Bionik slide agglutination test kit, Iran); the measured CRP levels were 6, 12, 24, 48, and 96 mg/L. ESR was measured by auto-analyzer (Electa Co., USA) and WBC count was performed by SysmecSE 9000 analyzer (Kolbe, Japan). The Tripticase soy broth and brain-heart-infusion broth were used as the growth media. They were transferred to chocolate agar after 24 hours, 48 hours, and seven days. Biochemical test was performed to determine the species.
3.3. Data Analysis
Statistical analysis was performed using SPSS for windows (version 18, SPSS Inc., Chicago, IL, USA). The normally distributed variances were expressed as mean ± SD. Student’s t-test was used to compare the mean values between the two groups. The data that were not normally distributed were expressed as medium and analyzed using the rank sum test. Unordered categorical variables were expressed as percentage, and the difference in proportion between the two groups was analyzed using the chi-squared test. A receiver operating characteristic (ROC) curve was employed to evaluate the effects of suPAR, WBC, ESR, and CRP levels on sepsis diagnosis.
4. Results
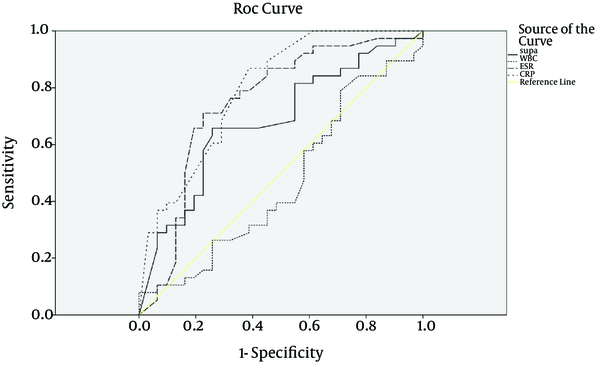
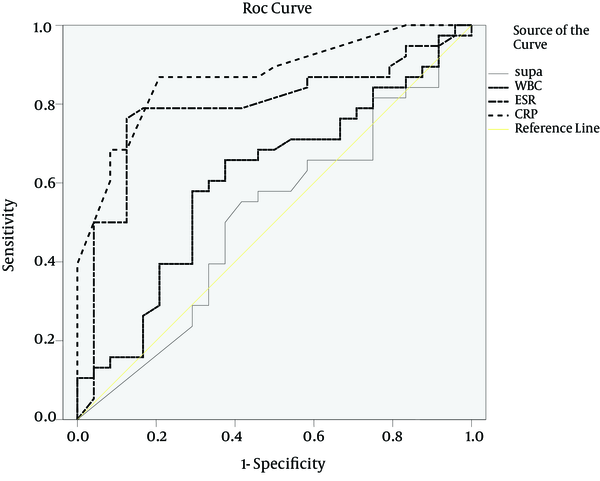
Among the patients in group B (with noninfectious SIRS) and C (non-SIRS), the central nervous system diseases (n = 19) was the most frequent diseases, followed by respiratory diseases (n = 17), malignant diseases (n = 10), multiple trauma (n = 8), cardiovascular diseases (4), gastroenterological diseases (n = 3), renal diseases (n = 2), and hematological diseases (n = 1). The patients' baseline data at the admission time are shown in Table 1. Temperature and APACHE IV scores were notably higher in the sepsis group than in the SIRS and control groups (P < 0.002). No statistical difference was noted for age or gender among the three groups. suPAR, ESR, and CRP levels on the day of ICU admission are shown in Table 1. These values were significantly higher in the sepsis group than in the SIRS and control groups (10 vs. 6.15 and 9.3 ng/mL, P < 0.05, 54.3 vs. 30.4 and 26.3 mm/h, P < 0.00, and 48 vs. 13 and 13.5 mg/L, P < 0.00, respectively). On the other hand, there was no difference in WBC count (13198 vs. 11926 and 10166 cell/µL, respectively; P = 0.107). The ROC curves were obtained for suPAR, CRP, ESR, and APACHE IV scores, which were significantly different between the sepsis and SIRS groups (Figure 1). The area under the curve (AUC) for suPAR, CRP, ESR, and APACHE IV scores were 0.68 (95% CL, 0.55-0.81), 0.79 (95% CL, 0.69-0.90), 0.75 (95% CL, 0.62-0.87), and 0.75 (95% CL, 0.62-0.87), respectively. When 8.45 ng/mL was set as the cut-off value for suPAR, the sensitivity was 0.66 and the specificity was 0.74 (Table 2). ROC curves were obtained for suPAR, CRP, ESR, WBC, and APACHE IV scores in the sepsis and non-SIRS groups (Figure 2). The AUC for CRP, ESR, and APACHE IV scores were 0.87 (95% CL, 0.78-0.96), 0.79 (95% CL, 0.67-0.91), and 0.73 (95% CL, 0.58-0.88), respectively. When 23 mg/L was set as the cut-off value for CRP, the sensitivity was 0.88 and the specificity was 0.88 and as 26 mm/h was set for ESR, the sensitivity was 0.78 and the specificity was 0.83 (Table 3). Soluble uPAR did not differ significantly in sepsis (A), noninfectious SIRS (B), and non-SIRS (C) groups aged under 65 (9.8 ± 7.5, 7.7 ± 5.2, and 11.2 ± 6.3 ng/mL, respectively) and in the patients over this age (11.2 ± 5.8, 8.3 ± 5.5, and 10.9 ± 7.2 ng/mL, respectively). Soluble uPAR did not differ significantly in sepsis (A), noninfectious SIRS (B), and non-SIRS (C) males (11.6 ± 6.1, 7.5 ± 5.6, and 10.6 ± 6.4 ng/mL, respectively) and females (9.9 ± 6.4, 8.6 ± 4.9, and 11.9 ± 6.4 ng/mL, respectively). A positive correlation was found between ESR and CRP (P < 0.01) and among APACHE IV,CRP and ESR (P < 0.01).
| Characteristic | Group A (n =40) | Group B ( n =43) | Group C (n =24) | P Value |
|---|---|---|---|---|
| Age, mean ± SD, y | 69.6 ± 18.2 | 55.65 ± 21.34 | 51.80 ± 24.78 | 0.46 |
| Gender, No. | 0.24 | |||
| Male | 26 | 25 | 16 | |
| Female | 14 | 18 | 8 | |
| Temperature, mean ± SD, ˚C | 38.3 ± 0.8 | 37.8 ± 0.9 | 37.5 ± 0.7 | 0.000 |
| ESR, mean ± SD, mm/h | 54.3 ± 27.6 | 30.4 ± 27.6 | 26.3 ± 18 | 0.000 |
| WBC, mean ± SD, cells/μL | 13198 ± 7121 | 11926 ± 4440 | 10166 ± 3906 | 0.107 |
| Median CRP [range], mg/L | 48 [12-96] | 13 [4-96] | 13.5 [8-48] | 0.000 |
| Median APACHE IV [range] | 56 [34-109] | 37.5 [10-87] | 37 [16-76] | 0.002 |
| Median suPAR [range], ng/mL | 10 [0.30-20] | 6.15 [0.30-20] | 9.3 [0.70-20] | 0.05 |
Receiver Operating Characteristic Curve in the Diagnosis of Patients With Sepsis (A) vs. Patients With Noninfectious SIRS (B)
Receiver Operating Characteristic Curve in the Diagnosis of Patients With Sepsis (A) vs. Non-SIRS Patients (C)
Area Under the Receiver Operating Characteristic Curve as a Means of Differentiating Sepsis (Group A) From SIRS (Group B) a
| Variable | AUC | P Value | Asymptomatic 95% Confidence Interval | Cut Point | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||||
| suPAR | 0.68 | 0.01 | 0.55 | 0.81 | 8.45 | 0.66 | 0.74 | 0.50 | 0.55 |
| CRP | 0.79 | 0.00 | 0.69 | 0.90 | 23 | 0.87 | 0.59 | 0.70 | 0.80 |
| ESR | 0.75 | 0.00 | 0.62 | 0.87 | 41 | 0.71 | 0.76 | 0.77 | 0.68 |
| WBC | 0.46 | 0.61 | 0.32 | 0.61 | 11150 | 0.61 | 0.41 | 0.50 | 0.55 |
| APACHE IV | 0.75 | 0.00 | 0.62 | 0.87 | 46.5 | 0.73 | 0.72 | 0.73 | 0.72 |
Area Under Receiver Operating Characteristic Curve as a Means of Differentiating Sepsis (Group A) From Non SIRS (Group C) a
| Variable | AUC | P Value | Asymptomatic 95% Confidence Interval | Cut point | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||||
| suPAR | 0.51 | 0.89 | 0.36 | 0.66 | 9.5 | 0.58 | 0.54 | 0.64 | 0.41 |
| CRP | 0.87 | 0.00 | 0.78 | 0.96 | 23 | 0.88 | 0.88 | 0.87 | 0.79 |
| ESR | 0.79 | 0.00 | 0.67 | 0.91 | 26 | 0.78 | 0.83 | 0.9 | 0.64 |
| WBC | 0.61 | 0.15 | 0.46 | 0.75 | 11150 | 0.60 | 0.67 | 0.76 | 0.50 |
| APACHE IV | 0.73 | 0.00 | 0.58 | 0.88 | 46.5 | 0.72 | 0.61 | 0.73 | 0.61 |
5. Discussion
Serine protease plasmin plays a central part in extravascular as well as intravascular fibrinolysis, and more generally in extracellular matrix degradation which is an essential part of tissue remodeling. A crucial element in regulation of these processes is the proteolytic activation of plasminogen transformation to plasmin. Two types of plasminogen activators (PA) have been characterized; tissue type PA (tPU) and urokinase PA (uPA). The primary role of tPA is thought to be in fibrin dissolution and thrombolysis, while uPA is mainly involved in pericellular matrix degradation during tissue remodeling. The effect of uPA is intensified and localized through binding to a specific cell bound receptor (uPAR), which is expressed on a variety of cell types, including neutrophils, monocytes/macrophages and malignant cells. Plasminogen activation is influenced by inflammation, and specifically the proinflammatory cytokines interleukin 1 and tumor necrosis factor α induce the up-regulation of uPA and down-regulation of tPA. uPAR may be released from the cell surface by either cleavage of the glycolipid anchor by a phospholipase, or cleavage of the protein close to the anchor, thus forming a free soluble receptor (suPAR). suPAR is detected in low but fairly constant concentrations in plasma of healthy, normal people. Increased plasma concentrations of suPAR have been found in patients with advanced cancers of lung, breast, and colon and inflammatory rheumatic diseases (5) and also in staphylococcal and HIV infection (7, 8). suPAR not only is present in human plasma or serum, but can also be found in other body fluids, including urine, cerebrospinal fluid, as well as in pleural, pericardial, and peritoneal fluids (9, 10). In this study, we analyzed the value of suPAR and some inflammatory markers in early diagnosis of sepsis in a heterogeneous group of ICU patients. Backes et al. in their systemic review showed that systemic levels of suPAR were significantly higher in critically ill patients compared to healthy controls. However, the area under the receiver operating characteristic curve for suPAR to discriminate between nonseptic and septic ICU patients is reported to be poor. They also declined that compared to other frequently used biological markers including CRP, procalcitonin (PCT), and soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), suPAR added a little to the diagnostic process (11). Data obtained through our study were quite similar to this systemic review. Kofoed et al. found 35% sensitivity and 67% specificity for suPAR to diagnose sepsis in patients, although he found that CRP performed better than suPAR (6). CRP, an acute-phase protein, is synthesized in the liver following stimulation by various cytokines including tumor necrosis factor-α and interleukin-6 (12). An elevated blood CRP concentration is thought to be highly suggestive of bacterial infection (12), and CRP level has been described as a good early marker in many studies (13-15). These studies found sensitivities between 86% and 94.3% and specificities between 60% and 87.3% (6, 13). In our study, CRP and ESR performed better than suPAR in diagnosing infection and did almost the same in discriminating between SIRS and sepsis. In addition to infection, there are several other conditions that commonly lead to substantial changes in CRP concentrations. These include trauma, surgery, burns, tissue necrosis, immunologically mediated inflammatory diseases, crystal-induced inflammatory diseases, and advanced cancer (12). Same as CRP, increase of suPAR was also reported in the absence of infection in rheumatoid arthritis (5), and virtually in all human cancers, suggesting its possible clinical applications as diagnostic marker, predictive tool of survival or clinical response, and as a target for therapy and imaging (16-18). However, we recognized that our study also had limitations. We studied a mixed group of medical and surgical ICU patients. We used clinical criteria, so it might have been difficult to ascertain the exact cause of SIRS in all the patients. suPAR, ESR, and CRP levels are substantial values for early diagnosis of sepsis. ESR and CRP have the advantages of familiarity, simplicity and lower costs in comparison with suPAR. Therefore, it is recommended to use CRP and ESR for differentiating sepsis from SIRS and non-SIRS infections. Unfortunately, the sample size of this study was small; larger studies are thus needed to further evaluate the value of suPAR in diagnosis of sepsis.
References
-
1.
Bochud PY, Calandra T. Pathogenesis of sepsis: new concepts and implications for future treatment. BMJ. 2003;326(7383):262-6. [PubMed ID: 12560281].
-
2.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530-8. [PubMed ID: 12664219]. https://doi.org/10.1007/s00134-003-1662-x.
-
3.
Spapen HD, Hachimi-Idrissi S, Corne L, Huyghens LP. Diagnostic markers of sepsis in the emergency department. Acta Clin Belg. 2006;61(3):138-42. [PubMed ID: 16881563]. https://doi.org/10.1179/acb.2006.022.
-
4.
Dekkers PE, ten Hove T, te Velde AA, van Deventer SJ, van Der Poll T. Upregulation of monocyte urokinase plasminogen activator receptor during human endotoxemia. Infect Immun. 2000;68(4):2156-60. [PubMed ID: 10722614].
-
5.
Slot O, Brunner N, Locht H, Oxholm P, Stephens RW. Soluble urokinase plasminogen activator receptor in plasma of patients with inflammatory rheumatic disorders: increased concentrations in rheumatoid arthritis. Ann Rheum Dis. 1999;58(8):488-92. [PubMed ID: 10419867].
-
6.
Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, et al. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007;11(2):R38. [PubMed ID: 17362525]. https://doi.org/10.1186/cc5723.
-
7.
Molkanen T, Ruotsalainen E, Thorball CW, Jarvinen A. Elevated soluble urokinase plasminogen activator receptor (suPAR) predicts mortality in Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2011;30(11):1417-24. [PubMed ID: 21479972]. https://doi.org/10.1007/s10096-011-1236-8.
-
8.
Lawn SD, Myer L, Bangani N, Vogt M, Wood R. Plasma levels of soluble urokinase-type plasminogen activator receptor (suPAR) and early mortality risk among patients enrolling for antiretroviral treatment in South Africa. BMC Infect Dis. 2007;7:41. [PubMed ID: 17509133]. https://doi.org/10.1186/1471-2334-7-41.
-
9.
Mizukami IF, Faulkner NE, Gyetko MR, Sitrin RG, Todd RF, 3rd. Enzyme-linked immunoabsorbent assay detection of a soluble form of urokinase plasminogen activator receptor in vivo. Blood. 1995;86(1):203-11. [PubMed ID: 7795225].
-
10.
Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27(3):157-72. [PubMed ID: 19893210]. https://doi.org/10.3233/DMA-2009-0657.
-
11.
Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38(9):1418-28. [PubMed ID: 22706919]. https://doi.org/10.1007/s00134-012-2613-1.
-
12.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448-54. [PubMed ID: 9971870]. https://doi.org/10.1056/NEJM199902113400607.
-
13.
Lobo SM, Lobo FR, Bota DP, Lopes-Ferreira F, Soliman HM, Melot C, et al. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123(6):2043-9. [PubMed ID: 12796187].
-
14.
Sierra R, Rello J, Bailen MA, Benitez E, Gordillo A, Leon C, et al. C-reactive protein used as an early indicator of infection in patients with systemic inflammatory response syndrome. Intensive Care Med. 2004;30(11):2038-45. [PubMed ID: 15378239]. https://doi.org/10.1007/s00134-004-2434-y.
-
15.
Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care. 2004;8(4):R234-42. [PubMed ID: 15312223]. https://doi.org/10.1186/cc2877.
-
16.
Boonstra MC, Verspaget HW, Ganesh S, Kubben FJ, Vahrmeijer AL, van de Velde CJ, et al. Clinical applications of the urokinase receptor (uPAR) for cancer patients. Curr Pharm Des. 2011;17(19):1890-910. [PubMed ID: 21711239].
-
17.
Jo M, Thomas KS, Wu L, Gonias SL. Soluble urokinase-type plasminogen activator receptor inhibits cancer cell growth and invasion by direct urokinase-independent effects on cell signaling. J Biol Chem. 2003;278(47):46692-8. [PubMed ID: 12963722]. https://doi.org/10.1074/jbc.M308808200.
-
18.
Mizukami IF, Todd RF, 3rd. A soluble form of the urokinase plasminogen activator receptor (suPAR) can bind to hematopoietic cells. J Leukoc Biol. 1998;64(2):203-13. [PubMed ID: 9715260].