Abstract
Background:
Carbapenem resistance due to acquired metallo-beta-lactamases (MBLs) is considered to be more serious than other resistance mechanisms.Objectives:
The aim of this study was to examine the effects of the methanolic extracts of Zataria multiflora, Ziziphus, Chamomile and Myrtus communis leaves on IMP-type MBL- producing Pseudomonas aeruginosa strains.Materials and Methods:
This cross-sectional descriptive study was conducted on burn patients hospitalized in Shahid Motahari Hospital, Tehran, Iran, during 2012 - 2013. Antibiotics and extracts susceptibility tests were performed using the disc diffusion and broth micro dilution methods. The metallo-beta-lactamase detection was performed by combination disk diffusion test. The bla (VIM) and bla (IMP) genes were detected by polymerase chain reaction (PCR) and sequencing methods.Results:
Eighty-three out of 96 samples were imipenem-resistant P. aeruginosa strains. Among 83 imipenem-resistant P. aeruginosa strains, 48 (57.9%) were MBL producers. Polymerase chain reaction and sequencing methods proved that these isolates were positive for blaIMP-1 genes, whereas none were positive for bla (VIM) genes. The minimum inhibitory concentration (MIC) for imipenem was 128 (µg/mL) for all strains. The MIC and minimum bactericidal concentration (MBC) of M. communis were 6.25 and 12.5 (mg/mL) for all isolates, respectively; the MIC and MBC of Z. multiflora were somehow the same. Methanolic extract of Chamomile showed to have a beneficial effect on this strain, while the Ziziphus leaves methanolic extract showed no significant effect on these isolates.Conclusions:
The results of this study reveal that the M. communis extract and methanolic extract of Chamomile have a high antibacterial effect on regular and IMP-producing P. aeruginosa strains; so, these extracts can be suitable alternatives for less-effective antibiotics, which are commonly used.Keywords
1. Background
Pseudomonas aeruginosa is an important nosocomial pathogen. In recent decades, inappropriate use of antibiotics has led to drug resistance among bacteria, which is the grounds of high mortality rates throughout the world, particularly among people with suppressed immunity. The production of metallo-β-lactamases (MBLs) that confer resistance to all β-lactams except aztreonam is a mechanism of increasing clinical importance, mostly driven by the international spread of MBL producing organisms. Furthermore, the MBL-encoding genes that located on integrons can be disseminated easily from one bacterium to another. Many MBLs have been found in P. aeruginosa, including Australian imipenemase (AIM), (Verona integron-encoded metallo-β-lactamases (VIM)), Sao Paolo metallo (SPM), Seoul imipenemase (SIM), German imipenemase (GIM), Japan, Kyorin university hospital imipenemase (KHM), New-Delhi metallo-beta-lactamase-1 (NDM-1) and imipenemase (IMP). The genes of both IMP and VIM-type in clinical isolates of P. aeruginosa are usually encoded on mobile elements inserted into class 1 integrons. The integrons are located on transposons or plasmids, the distribution of which contributes to the wide spread of this resistance mechanism (1-4).
Zataria multiflora is a member of the Labiatae with a Woody, fibrous root, and its leaves are small, narrow, and elliptical, greenish-gray in colors. It grows in countries like Pakistan, Afghanistan and Iran. Traditionally, it has been utilized as treatment of sore throat, jaundice, chronic catharsis and asthma. Z. multiflora has been reported to have applied for medical properties including pain-relieving, immunostimulant, and antibacterial, anticandidal, antifungal and anti-inflammatory effects (4-6).
The genus Ziziphus belongs to the Rhamnaceae family. The members of this genus are drought tolerant and very resistant to heat. It is a small to medium-sized tree, with a spreading canopy. It has widely extended from South Africa northwards to Ethiopia and Arabia. The leaves of the plant are utilized in the treatment of diarrhea, wounds, abscesses, swelling and gonorrhea and they are also used in the treatment of liver diseases, asthma and fever (7, 8).
Chamomile is a member of the daisy family (Asteraceae or Compositae). It is a perennial herbaceous plant cultivated in western Europe and north Africa. Inward in traditional medicine, Chamomile is applied as an anti-inflammatory agent for stomach upsets. In women, the antispasmodic effects of Chamomile ease menstrual cramps, and lessen the possibility of premature labor also, Chamomile extract’s stimulating effect on leukocytes (macrophages and b lymphocytes) and it is applied in skin irritations and eczema (9, 10).
Myrtle (Myrtus communis l.) is an evergreen shrub that belongs to the family of Mirtaceae that grows spontaneously. It is still extensively cultivated throughout the Mediterranean area. In classic medicine, myrtle has been shown to have anti-inflammatory effects. The anti-microbial activity of myrtle in Escherichia coli, staphylococcus aureus, P. aeruginosa, Proteus Vulgaris, Proteus Mirabilis, Klebsiella aerogenes, salmonella typhi and Shigiella has been determined (11, 12).
2. Objectives
The aim of this study was to define the antibiotic resistance patterns of P. aeruginosa, detect blaVIM and blaIMP MBL genes, and lastly evaluate the effects of the methanolic extracts of the leaves of Z. Multiflora, Ziziphus, Chamomile and M. communis on P. aeruginosa strains producing MBL (blaimp) isolated from the burn patients hospitalized in Shahid Motahari hospital, Tehran, Iran during 2011 - 2012.
3. Materials and Methods
3.1. Sampling Size
This is the random sampling, and the number of isolation was selected for this study according to the following Equation 1 (P = 0.5; d = 0.1):
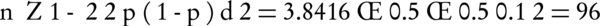
Data were analyzed using the chi-square, t-test, Fisher’s exact test with SPSS software version 16 (SPSS Inc USA). P values of less than 0.05 were considered statistically significant.
3.2. Isolation and Clinical Identification
Ninety-six P. aeruginosa strains were isolated from 400 burn patients (men and women) referred to Shahid Motahari hospital (level I burn care center in Tehran, this is the main general hospital for the burning patients and also it is the main center for the burning research in Tehran, Iran since February 2012 till October 2013. Most of the samples were isolated during spring and summer due to the prevalence of burn patients in these seasons. Also, the higher rates of P. aeruginosa infection were observed during the hot months.
To prepare the samples, the wounds were washed with physiological serum. At first samples were transferred to culture media such as Cetrimid and MacConkey agar then incubated at 37°C for 24 hours. Then, we used biochemical tests including oxides, catalase, and growth ability at 42°C. Pseudomonas aeruginosa ATCC27853 was used as a control strain.
3.3. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility to imipenem (IPM, 10 μg), meropenem (MEM, 10 μg), ceftazidime (CAZ, 30 μg), cefotaxime (CTX, 30 μg), amikacin (AK, 30 μg), tobramycin (TOB, 10 μg), piperacillin/Tazobactam (PTZ, 100/10 μg), ciprofloxacin (CIP, 5 μg), cefepime (FEP, 30 μg), ceftriaxone (CRO, 30 μg), aztreonam (ATM, 30 μg), gentamicin (GEN,10 μg) and carbenicillin (Car,100 μg) (MastGroup, Merseyside, UK) was tested on the isolated P. aeruginosa samples, as well as the control ATCC27853 according to clinical laboratory standards institute (CLSI) guidelines.
3.4. Molecular Detection Methods
blaIMP and blaVIM genes were detected by polymerase chain reaction (PCR) method and DNA templates were prepared by Qiagene kit. The polymerase chain reaction amplification for blaIMP and blaVIM was performed with primers VIM-F (5′-GTTTGGTCGCATATCGCAAC-3′) and VIM-R (5′-AATGCGCAGCACCAGGATAG-3′) for blaVIM gene and primers IMP-F (5′GAAGGCGTTTATGTTCATAC-3′) and IMP-R (5′GTATGTTTCAAGAGTGATGC-3′).
3.4.1. Sequencing
The PCR purification and the sequencing were performed at the same company. (Bioneer Co., Korea) .The sequences were analyzed with Chromas 1.45 and MEGA- 4 softwares and BLAST at PubMed NCBI.
3.5. Plant Materials
The leaves of Z. multiflora, Ziziphus, Chamomile and M. communis plants were collected from the Fars Province in Iran, during 2012. The leaves of the plants were dried at 25ºC and then powdered using a mechanical grinder. Ten gram of each powder sample was soaked in 100 mL of methanol (96%, v/v) from (Merck, Germany). The mixture is putted for 48 hours in a dry place. The solution was filtered at first by Whatman No. 1 filter paper to clarify and then through a 0.45 μm membrane filter. Then, it was filtered through a filter paper slowly. Extracts obtained separately were poured into Petri dishes and dried in laboratory space.
4. Results
From a total of 400 patients, 96 P. aeruginosa strains were isolated, that 83 were resistant to imipenem and ceftazidime. The combination disk diffusion test showed that among the 83 imipenem which are non-susceptible P. aeruginosa strains, 48 (57.9%) were MBL producers. All MBL-producing P. aeruginosa strains were resistant to meropenem, imipenem, ceftazidime, amikacin, tobramycin, ciprofloxacin, aztreonam, piperacillin/tazobactam, ceftriaxone, cefepime and carbenicillin; while 49% of isolates were resistant to gentamicin, indicating that 100% of isolates were multi-drug resistant (MDR) (resistance to more than three antibiotics from different classes was defined as MDR). The minimum inhibitory concentration (MIC) of different antibiotics for IMP-producing P. aeruginosa strains is shown in Table 1. Using the PCR method, 6 isolates were positive for bla (IMP) gene, while bla (VIM) gene was not detected. Sequencing of PCR products showed a conserved region of the restriction sequence blaIMP-1 gene that was confirmed by the BLAST. Forty-eight patients (57.9%) were infected with MBL-producing Pseudomonas strains, of whom 4 (8.3%) died. The antibacterial potency of Z. multiflora, Ziziphus, Chamomile and M. communis extracts against six IMP-producing P. aeruginosa strains were evaluated by the microdilution method as described by CLSI. The results of MICs and MBCs (mg/mL) of Chamomile and M. communis against IMP-producing P. aeruginosa strains have presented in Table 2, while Z. multiflora, Ziziphus did not show any significant effect on these 6 isolates.
Distribution of Minimum Inhibitory Concentrations of Antibiotics for IMP-Producing Pseudomonas aeruginosa Strains
| Antibiotics | Minimum Inhibitory Concentration, µg/mL | |||||
|---|---|---|---|---|---|---|
| P. a FSH2IMP | P. a FSH22IMP | P. a FSH28IMP | P. a FSH40IMP | P. a FSH42IMP | P. a FSH47IMP | |
| Imipenem | 128 | 128 | 128 | 128 | 128 | 128 |
| Meropenem | 64 | 64 | 64 | 64 | 64 | 64 |
| Cefepime | 128 | 128 | 128 | 128 | 128 | 128 |
| Ceftazidime | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 |
| Cefotaxime | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 |
| Ampicillin | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 |
| Piperacillin/Tazobactam | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 |
| Ceftriaxone | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 | ≥ 256 |
Frequency of Minimum Inhibitory Concentrations of Myrtus communis, Zatariamultiflora, Ziziphus and Chamomile Extracts for IMP-Producing Pseudomonas aeruginosa Strains
| Strain | Myrtus communis | Zataria multiflora | Chamomile | Ziziphus | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| P.a FSH2IMP | 6.25 | 12.5 | 6.25 | 12.5 | 12.5 | 15 | 12.5 | 25 |
| P.a FSH22IMP | 6.25 | 12.5 | 6.25 | 12.5 | 12.5 | 25 | 12.5 | 25 |
| P.a FSH28IMP | 6.25 | 12.5 | 6.25 | 6.25 | 12.5 | 25 | 12.5 | 25 |
| P.a FSH40IMP | 6.25 | 12.5 | 6.25 | 12.5 | 12.5 | 25 | 12.5 | 25 |
| P.a FSH42IMP | 6. 25 | NA | 6.25 | 6.25 | 12.5 | 25 | 12.5 | 25 |
| P.a FSH47IMP | 6.25 | NA | 6.25 | 6.25 | 12.5 | 25 | 12.5 | 25 |
| P.aeruginosa ATCC27853 | 6.25 | 12.5 | 1.56 | NA | 12.5 | 25 | 12.5 | 25 |
5. Discussion
Pseudomonas aeruginosa is an opportunistic pathogen and one of the most important causes of infection in burn patients that followed by Staphylococcus aureus and Acinetobacter baumannii (13). Pseudomonas aeruginosa acquired antibiotic resistance; so, we need new methods of treatment to decrease the probability of drug resistance. MBL producer P aeruginosa in burn patients is the main reasons for increasing mortality and morbidity rates. In the two last decades, P aeruginosa was the most dominant bacteria in burn patients in Tehran, Iran (14). All of the P. aeruginosa strains in our study have resistance against almost all antibacterial agents. The MBL-producing P aeruginosa strains were resistant to amikacin, ciprofloxacin, ceftazidime, tobramycin, imipenem, meropenem, ceftriaxone, carbenicillin, piperacillin/tazobactam and cefepime. Also, 49% of the isolates were resistant to gentamycin. P aeruginosa have some mechanisms that can cause drug resistance such as enzyme mechanism and Efflux pump iron which have the ability to develop resistance to antibacterial agents (15). We have a high rate of MBLs in our study in comparison to some studies in other parts of the world, the study in Spain showed that just 6.9% of isolates were MBL producer (16), in India MBL producer were 33% (17), but the rate of MBL producer was lower in our study, maybe because of treatment policy such as antibiotics that prescribed and hospitalization condition. The most reported, as well as in Iran indicated that the prevalence of VIM beta-lactamase is more than IMP (18, 19) but in our study IMP was the most dominant MBL that is in concordance with other studies. In our study, 6 isolates were positive for bla (IMP) gene. Some other genes probably can cause resistance such as GIM, KHM, SIM, AIM, SPM, NDM and FIM (20-22). The mortality rate of infection due to MBL-producer P aeruginosa in Spain was 27% (23), in Brazil was 82.6% (24) and in our study was 8.3%. Also, VIM-2 can cause drug resistance; the existence of this gene in P. aeruginosa was reported in France for the first time (25). In our study, the antibacterial effect of, Z. multiflora plants and M. communis, Chamomile, Ziziphus leaves were tested against MBL-producer P. aeruginosa. We conclude that Myrtus communis extracts had a beneficial antibacterial effect against regular and IMP-producing P. aeruginosa strains. Kang et al. used ethanolic extracts of M. communis and inhibitory growth of P. aeruginosa was observed (26). Akin et al. concluded that M. communis essential oil was not a good inhibitor for P. aeruginosa; however, we found M. communis as a good inhibitor in our study (27). Owlia et al. has shown that M. communis had an antibacterial effect on this isolates, but Chamomilla essential oils were effortless on Pseudomonas aeruginosa that is in contrast to our study (28). Hashemi et al. reported that the inhibitory effect of Z. multiflora on P. aeruginosa isolated from burn patients are more than Peganum harmala and M. communis (29). In the same study to our research Al-Saimary et al. in 2001 in Iraq found that the aqueous extracts of M. communis and Eucalyptus leaves had a good effect on P aeruginosa that isolated from burned patients (30). Bokaeian et al. in 2014 suggested that M. communis leaves are powerful bactericidal and effective against P. aeruginosa and Klebsiella pneumonia (31). Carvalho et al. reported that ethanolic extract of Chamomile had a beneficial antibacterial effect against P. aeruginosa and no effect against S. aureus, E. coli, Salmonella enterica subsp. enterica sorovar Typhimurium (32).
5.1. Conclusions
As the IMP producing P. aeruginosa is increasing in burn patients, detection of them is absolutely important to identify P. aeruginosa drug resistance, which can show and improve developing methods of drug therapy as alternative models for the physicians to avoid synthetic resistance drugs for patient treatment. The methanolic extract of Z. multiflora and M. communis had more beneficial effect on clinical IMP-producing P. aeruginosa strains compared to the routine approach in this bacteria treatment. Therefore, these herbal extracts can be the best alternatives for the traditionally less-effective antibiotics, which are normally used till now.
Acknowledgements
References
-
1.
Willmann M, Kuebart I, Marschal M, Schroppel K, Vogel W, Flesch I, et al. Effect of metallo-beta-lactamase production and multidrug resistance on clinical outcomes in patients with Pseudomonas aeruginosa bloodstream infection: a retrospective cohort study. BMC Infect Dis. 2013;13:515. [PubMed ID: 24176052]. https://doi.org/10.1186/1471-2334-13-515.
-
2.
Kali A, Srirangaraj S, Kumar S, Divya HA, Kalyani A, Umadevi S. Detection of metallo-beta-lactamase producing Pseudomonas aeruginosa in intensive care units. Australas Med J. 2013;6(12):686-93. [PubMed ID: 24391679]. https://doi.org/10.4066/AMJ.2013.1824.
-
3.
Doosti M, Ramazani A, Garshasbi M. Identification and characterization of metallo-beta-lactamases producing Pseudomonas aeruginosa clinical isolates in University Hospital from Zanjan Province, Iran. Iran Biomed J. 2013;17(3):129-33. [PubMed ID: 23748890].
-
4.
Fallah F, Taherpour A, Borhan RS, Hashemi A, Habibi M, Sajadi Nia R. Evaluation of Zataria multiflora Boiss and Carum copticum antibacterial activity on IMP-type metallo-beta-lactamase-producing Pseudomonas aeruginosa. Ann Burns Fire Disasters. 2013;26(4):193-8. [PubMed ID: 24799849].
-
5.
Mohammadi A, Gholamhoseinian A, Fallah H. Zataria multiflora increases insulin sensitivity and PPARgamma gene expression in high fructose fed insulin resistant rats. Iran J Basic Med Sci. 2014;17(4):263-70. [PubMed ID: 24904719].
-
6.
Boskabady MH, Kaveh M, Eftekhar N, Nemati A. Zataria multiflora Boiss and Carvacrol Affect beta(2)-Adrenoceptors of Guinea Pig Trachea. Evid Based Complement Alternat Med. 2011;2011:857124. [PubMed ID: 21151671]. https://doi.org/10.1155/2011/857124.
-
7.
Olajuyigbe OO, Afolayan AJ. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. BMC Complement Altern Med. 2011;11:130. [PubMed ID: 22176659]. https://doi.org/10.1186/1472-6882-11-130.
-
8.
Dahiru D, William ET, Nadro MS. Protective effect of Ziziphus mauritiana leaf extract on carbon tetrachloride-induced liver injury. Afr J Biotechnol. 2005;4(10):1177-9.
-
9.
Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with a bright future (Review). Mol Med Rep. 2010;3(6):895-901. https://doi.org/10.3892/mmr.2010.377.
-
10.
Farideh ZZ, Bagher M, Ashraf A, Akram A, Kazem M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J Reprod Infertil. 2010;11(3):169-74. [PubMed ID: 23926485].
-
11.
Zanetti S, Cannas S, Molicotti P, Bua A, Cubeddu M, Porcedda S, et al. Evaluation of the Antimicrobial Properties of the Essential Oil of Myrtus communis L. against Clinical Strains of Mycobacterium spp. Interdiscip Perspect Infect Dis. 2010;2010. [PubMed ID: 20706606]. https://doi.org/10.1155/2010/931530.
-
12.
Minaei MB, Ghadami Yazdi E, Ebrahim Zadeh Ardakani M, Hashem Dabaghian F, Ranjbar AM, Rastegari M, et al. First Case Report: Treatment of the Facial Warts by Using Myrtus communis L. Topically on the Other Part of the Body. Iran Red Crescent Med J. 2014;16(2). eee13565. [PubMed ID: 24719732]. https://doi.org/10.5812/ircmj.13565.
-
13.
Anvarinejad M, Japoni A, Rafaatpour N, Mardaneh J, Abbasi P, Amin Shahidi M, et al. Burn Patients Infected With Metallo-Beta-Lactamase-Producing Pseudomonas aeruginosa: Multidrug-Resistant Strains. Arch Trauma Res. 2014;3(2). eee18182. [PubMed ID: 25147779]. https://doi.org/10.5812/atr.18182.
-
14.
Estahbanati HK, Kashani PP, Ghanaatpisheh F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns. 2002;28(4):340-8. [PubMed ID: 12052372].
-
15.
Cornaglia G, Giamarellou H, Rossolini GM. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect Dis. 2011;11(5):381-93. [PubMed ID: 21530894]. https://doi.org/10.1016/S1473-3099(11)70056-1.
-
16.
Oglesby-Sherrouse AG, Djapgne L, Nguyen AT, Vasil AI, Vasil ML. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog Dis. 2014;70(3):307-20. [PubMed ID: 24436170]. https://doi.org/10.1111/2049-632X.12132.
-
17.
Riera E, Cabot G, Mulet X, Garcia-Castillo M, del Campo R, Juan C, et al. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J Antimicrob Chemother. 2011;66(9):2022-7. [PubMed ID: 21653605]. https://doi.org/10.1093/jac/dkr232.
-
18.
De AS, Kumar SH, Baveja SM. Prevalence of metallo-b-lactamase producing Pseudomonas aeruginosa and Acinetobacter species in intensive care areas in a tertiary care hospital. Indian J Crit Care Med. 2010;14(4):217. https://doi.org/10.4103/0972-5229.76089.
-
19.
Ciofi Degli Atti M, Bernaschi P, Carletti M, Luzzi I, Garcia-Fernandez A, Bertaina A, et al. An outbreak of extremely drug-resistant Pseudomonas aeruginosa in a tertiary care pediatric hospital in Italy. BMC Infect Dis. 2014;14:494. [PubMed ID: 25209325]. https://doi.org/10.1186/1471-2334-14-494.
-
20.
Aghamiri S, Amirmozafari N, Fallah Mehrabadi J, Fouladtan B, Samadi Kafil H. Antibiotic Resistance Pattern and Evaluation of Metallo-Beta Lactamase Genes Including bla- IMP and bla- VIM Types in Pseudomonas aeruginosa Isolated from Patients in Tehran Hospitals. ISRN Microbiol. 2014;2014:941507. [PubMed ID: 24944839]. https://doi.org/10.1155/2014/941507.
-
21.
Pollini S, Maradei S, Pecile P, Olivo G, Luzzaro F, Docquier JD, et al. FIM-1, a new acquired metallo-beta-lactamase from a Pseudomonas aeruginosa clinical isolate from Italy. Antimicrob Agents Chemother. 2013;57(1):410-6. [PubMed ID: 23114762]. https://doi.org/10.1128/AAC.01953-12.
-
22.
Borra PS, Samuelsen O, Spencer J, Walsh TR, Lorentzen MS, Leiros HK. Crystal structures of Pseudomonas aeruginosa GIM-1: active-site plasticity in metallo-beta-lactamases. Antimicrob Agents Chemother. 2013;57(2):848-54. [PubMed ID: 23208706]. https://doi.org/10.1128/AAC.02227-12.
-
23.
Pena C, Suarez C, Tubau F, Gutierrez O, Dominguez A, Oliver A, et al. Nosocomial spread of Pseudomonas aeruginosa producing the metallo-beta-lactamase VIM-2 in a Spanish hospital: clinical and epidemiological implications. Clin Microbiol Infect. 2007;13(10):1026-9. [PubMed ID: 17651449]. https://doi.org/10.1111/j.1469-0691.2007.01784.x.
-
24.
Marra AR, Pereira CA, Gales AC, Menezes LC, Cal RG, de Souza JM, et al. Bloodstream infections with metallo-beta-lactamase-producing Pseudomonas aeruginosa: epidemiology, microbiology, and clinical outcomes. Antimicrob Agents Chemother. 2006;50(1):388-90. [PubMed ID: 16377720]. https://doi.org/10.1128/AAC.50.1.388-390.2006.
-
25.
Poirel L, Naas T, Nicolas D, Collet L, Bellais S, Cavallo JD, et al. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother. 2000;44(4):891-7. [PubMed ID: 10722487].
-
26.
Kang CG, Hah DS, Kim CH, Kim YH, Kim E, Kim JS. Evaluation of antimicrobial activity of the methanol extracts from 8 traditional medicinal plants. Toxicol Res. 2011;27(1):31-6. [PubMed ID: 24278548]. https://doi.org/10.5487/TR.2011.27.1.031.
-
27.
Akin M, Aktumsek A, Nostro A. Antibacterial activity and composition of the essential oils of Eucalyptus camaldulensis Dehn. and Myrtus communis L. growing in Northern Cyprus. Afr J Biotechnol. 2012;9(4).
-
28.
Owlia P, Saderi H, Rasooli I, Sefidkon F. Antimicrobial characteristics of some herbal Oils on Pseudomonas aeruginosa with special reference to their chemical compositions. Iranian J Pharm Res. 2010:107-14.
-
29.
Hashemi A, Shams S, Barati M, Samedani A. Antibacterial effects of methanolic extracts of Zataria multiflora, Myrtus communis and Peganum harmala on Pseudomonas aeruginosa producing ESBL. Arak Med Univ J. 2011;14(4):104-12.
-
30.
Al-Saimary IE, Bakr SS, Jaffar T, Al-Saimary AE, Salim H, Al-Muosawi R. Effects of some plant extracts and antibiotics on Pseudomonas aeruginosa isolated from various burn cases. Saudi Med J. 2002;23(7):802-5. [PubMed ID: 12174229].
-
31.
Bokaeian M, Amini-Boroujeni N, Sahi Z, Saeidi S. Antibacterial activities of Myrtus communis L extract against multi-drug resistant Klebsiella pneumonia and Pseudomonas aeruginosa. Int J Biosci. 2014;4(9):254-9.
-
32.
Carvalho AF, Silva DM, Silva TRC, Scarcelli E, Manhani MR. Evaluation of the antibacterial activity of ethanolic and cyclohexane extracts of chamomile flowers (Matricaria chamomilla L.). Revista Brasileira de Plantas Medicinais. 2014;16(3):521-6.