1. Background
Ventilator associated pneumonia (VAP) is one of the most important nosocomial infections with often a poor outcome and heavy economic burden on health care systems due to increased mechanical ventilation and intensive care unit (ICU) stay days as well as mortality rates (1-3). According to previous studies, it is estimated that 3.5% of patients hospitalized for three or more days developed pneumonia (4) and prolonged ventilator dependency results in increased VAP incidence and consequent mortality (5, 6). Its cardinal signs include fever, incidence of or increase in purulent secretions, leukocytosis, oxygenation worsening, new or progressive lung infiltrates on chest imaging, and cultures of secretions revealing pathogenic organisms (7, 8). Most common pathogens are Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella spp., and Acinetobacter spp. drug resistance is common. A total of 50% of Staphylococcus aureus isolates are methicillin-resistant, 25% to 30% of Pseudomonas and Klebsiella isolates are ceftazidime and cefepime resistant, and 60% of Acinetobacter isolates are carbapenem resistant (9, 10).
There are some factors that make the patient vulnerable to develop nosocomial and ventilator-associated pneumonia. One of these important factors is the increase in the bacterial burden of the upper respiratory and orogastric tracts due to prolonged hospitalization, antibiotic exposure, and gastric acid suppression (4, 11-15). There are several studies that have been conducted among critically ill and mechanically ventilated patients for evaluating the effect of different gastrointestinal prophylactic agents, other than pantoprazole, on bleeding prevention, and nosocomial pneumonia occurrence (16-22).
Pantoprazole is a proton pump inhibitor (PPI) that is widely used in critically ill patients due to potent gastric acidity lessening and therefore, lowers gastrointestinal bleeding rate. For this reason, evaluating the incidence of gastrointestinal bleeding and ventilator-associated pneumonia in critically ill patients receiving pantoprazole and other agents of stress related mucosal disease (SRMD) prophylaxis, especially ranitidine (a histamine-2 blocker), has been of importance and value (23-26).
Some of the studies in this domain have been done by the authors of this article, for example evaluating the effect of ranitidine and pantoprazole on gastrointestinal bleeding prophylaxis in critically ill patients (23), investigating the predicted mortality of critically ill patients in the pantoprazole and ranitidine recipients (24), or comparing the effect of pantoprazole and ranitidine on VAP incidence (25). This study is along with the preceding ones and complementary of them, as we know, there is no study that has been done for evaluating the organisms responsible for VAP in critically ill patients admitted in the ICU, according to their SRMD prophylaxis regimen.
2. Objectives
In this study we aimed to compare the patients who had developed VAP during mechanical ventilation, in the organisms obtained from their secretions, based on SRMD prophylaxis they had received, either pantoprazole or ranitidine.
3. Methods
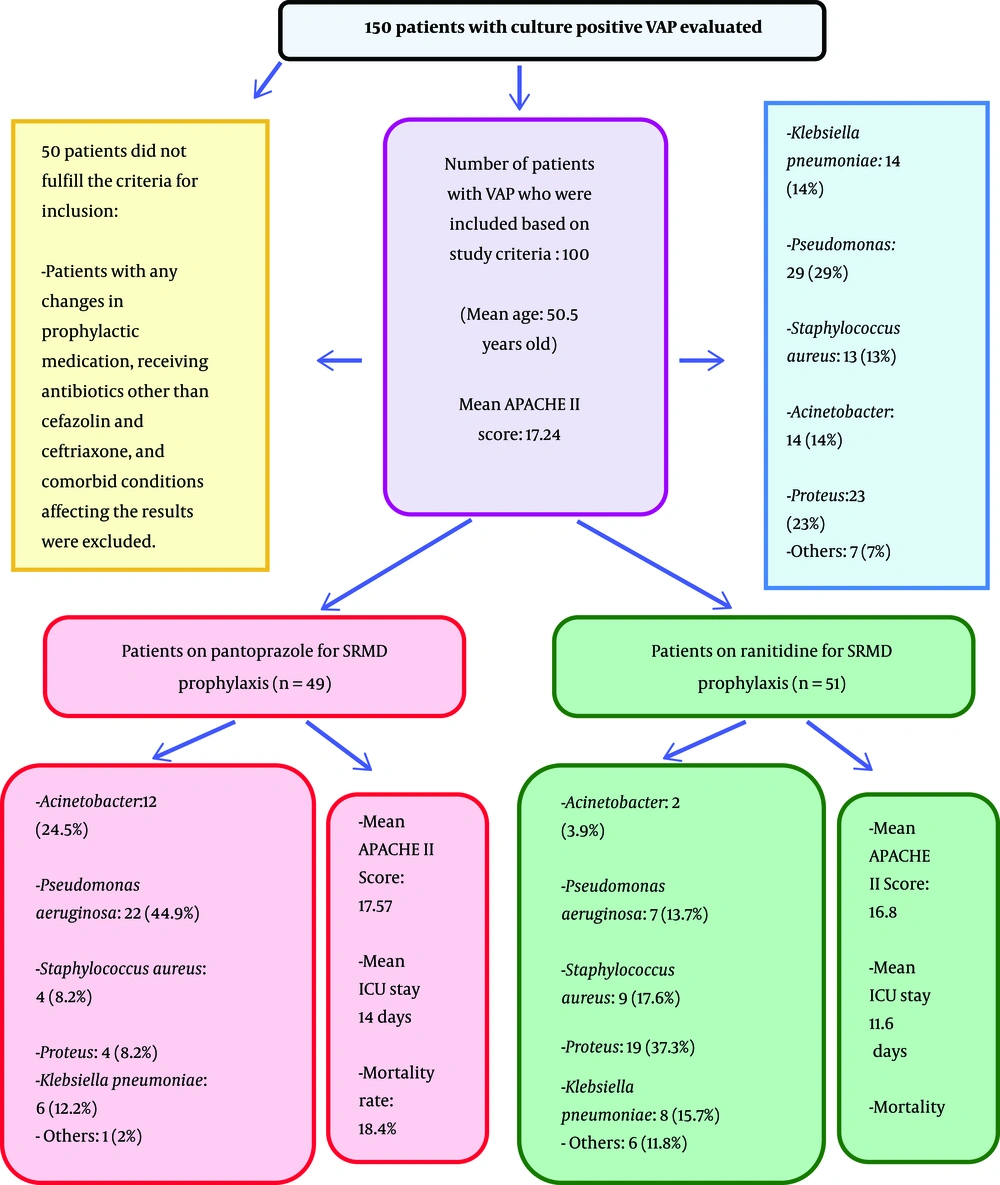
In this cross-sectional study that has been done in the ICU of Besat Hospital, Hamadan University of Medical Sciences, Hamadan, Iran, from May 2012 to May 2014, a total of 150 ICU patients who had been on a mechanical ventilation (either due to the need of respiratory support after surgery or trauma) and developed documented culture positive VAP were included. In this study, VAP was defined as an emergence of fever, purulent secretions (or increase in the amount of purulent secretions), leukocytosis, worsening in oxygenation, new or progressive lung infiltrates on chest radiograph or CT scan, and cultures of secretions revealing pathogenic organisms, with at least 48 hours of intubation. With this regard, previously healthy patients older than 18 years old, with a history of multiple trauma or surgery in whom intubation was necessary, and with a clinical pulmonary infection score (CPIS) equal and more than six, according to CPI scoring of Schurink et al., were included (27). APACHE II score, (which is one of the severity-of-disease classification systems for patients in ICU) and predicted mortality were calculated within the first 24 hours of ICU stay, and the patients with APACHE II score between 10 and 24 were included. A total of 100 patients who had been included and then divided into two groups based on SMRD prophylaxis were on either PPI or H2 blocker, on 40 mg of intravenous pantoprazole (NycomedExir Pharmaceutical Co., Broojerd, Iran) administered daily, or 50 milligrams of intravenous ranitidine (Kimi) administered three times daily for SRMD prophylaxis. The patients with any changes in SRMD prophylaxis during ICU stay were excluded. All secretion samples were obtained by mini-bronchoalveolar lavage (mini-BAL) and cultured in blood agar media; hose with contaminated samples were omitted. The patients with neutropenia, AIDS (acquired immunodeficiency syndrome), corticosteroid receipt, and any other conditions that predispose developing special nosocomial infections were excluded from the study. Patients receiving antibiotics other than those for surgical site infection prophylaxes (cefazolin and ceftriaxone) were excluded from the study. Finally, 100 patients who fulfilled all the criteria mentioned above were evaluated for the responsible organisms and type of SRMD prophylaxis they were on in order to conclude whether the type of SRMD prophylaxis promotes the growth of some organisms resulting in VAP or not. With respect to the type of prophylaxis received, the patients were categorized into two groups and compared. The flow of patients has been demonstrated in Figure 1.
Data analysis was done by using Student t test for normally distributed variables and Mann-Whitney U test for variables that were not normally distributed. The Chi2 and Fisher Exact tests were used to compare categorical variables as appropriate.
4. Results
Among these 100 VAP patients, 49 patients were on pantoprazole (49%) and 51 on ranitidine prophylaxiacinetobacs (51%) (Table 1). There were 59 men (59%) and 41 women (41%) ranging from 19 to 82 years old with the mean of 50.5 years (total); the mean age was 49 and 51 years old in pantoprazole and ranitidine group respectively, which were not significantly different (P = 0.586). APACHEII score was ranging between 15 and 21 with the mean of 17.57 in the pantoprazole group and 16.80 in the ranitidine group; with the P-value of 0.006 there was a significant difference between them statistically (P < 0.05), however, clinically, there was no significant difference among these two groups (statistically but not clinically different). The minimum ICU stay was six and the maximum was 25 days, with an average of 14.06 in pantoprazole group and 11.6 in ranitidine group (with P-value of 0.001, they were significantly different). Among the total of patients, 15 patients died (15%), nine from pantoprazole (9% of all) and six from ranitidine the group (6% of all); with a mortality rate of 18.4% and 11.8% within the pantoprazole and ranitidine group, respectively. The difference was statistically significant (P < 0.001). With regard to multi-drug resistant pathogens, there were 38 (38%) resistant organisms totally, 27 within the pantoprazole group (55.1%) and 11 in the ranitidine group (21.6%). This means that multi-drug resistant (MDR) pathogens have grown significantly higher beside pantoprazole prophylaxis for SRMD (P = 0.001) (Table 2 and Figure 2).
| Variables | Pantoprazole | Ranitidine | P-Value |
|---|---|---|---|
| Age (years) | 49.49 ± 17.6 | 51.49 ± 18.9 | 0.5 |
| Gender | 0.5 | ||
| Male | 29 (59.2) | 30 (58.8) | |
| Female | 20 (40.8) | 21 (41.2) | |
| Trauma | 33 (64.7) | 30 (61.2) | |
| APACHE II | 17.57 ± 1.52 | 16.8 ± 1.2 | 0.006b |
aValues are presented as No. (%) or Mean ± SD.
bStatistically significant.
| Variables | Pantoprazole | Ranitidine | P-Value |
|---|---|---|---|
| Pathogens | < 0.001b | ||
| Acinetobacter | 12 (24.5) | 2 (3.9) | |
| Pseudomonas aeruginosa | 22 (44.9) | 7 (13.7) | |
| Staphylococcus aureus | 4 (8.2) | 9 (17.6) | |
| Proteus | 4 (8.2) | 19 (37.3) | |
| Klebsiella | 6 (12.2) | 8 (15.7) | |
| Others | 1 (2) | 6 (11.8) | |
| MDR | 0.001b | ||
| Yes | 40 (78.4) | 11 (21.6) | |
| No | 22 (44.9) | 27 (55.1) | |
| ICU stay (days) | 14.06 ± 3.49 | 11.61 ± 3.83 | 0.001b |
| Hospital mortality | 0.3b | ||
| Yes | 9 (18.4) | 6 (11.8) | |
| No | 40 (81.6) | 45 (88.2) |
aValues are presented as No. (%) or Mean ± SD.
bStatistically significant.
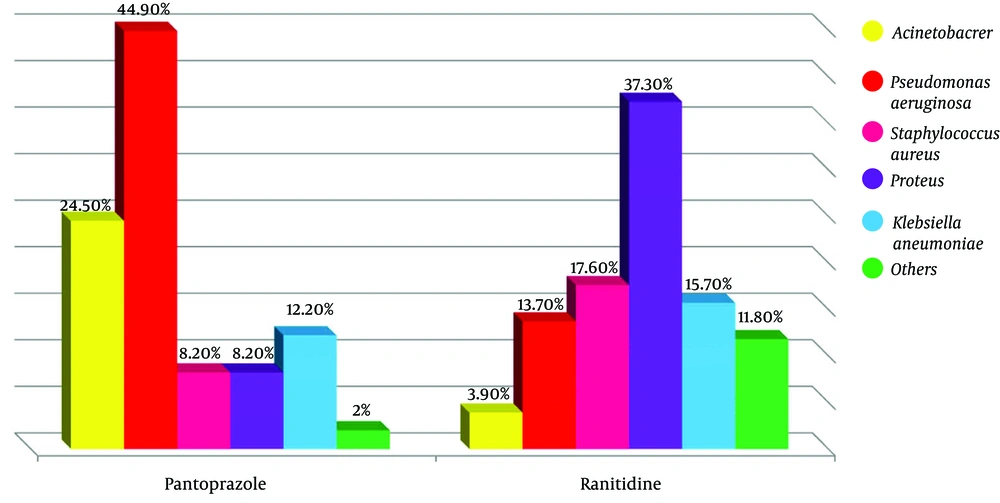
Among all the patients, 14 cultures were positive for Acinetobacter (14%), 29 for Pseudomonas aeruginosa (29%), 13 for Staphylococcus aureus (13%), 23 for Proteus (23%) and 14 for Klebsiella pneumoniae (14%). Other organisms (i.e. coagulase negative staphylococci, E. coli, or Enterobacter) contributed to seven of the total cultures (7%). In details, within the pantoprazole group, 12 patients had their secretions positive for Acinetobacter (24.5%), 22 for Pseudomonas aeruginosa (44.9%), four for Staphylococcus aureus (8.2%), four for Proteus (8.2%), and six for Klebsiella pneumoniae (12.2%). One patient had a positive culture for other organisms as mentioned above (2.0%). In the ranitidine group, the results included two Acinetobacter (3.9%), seven Pseudomonas aeruginosa (13.7%), nine Staphylococcus aureus (17.6%), 19 Proteus (37.3%), eight Klebsiella pneumoniae (15.7%), and six other organisms as defined above (11.8%).
There was a significant difference between the types of organisms obtained from two groups. Some bacteria have grown more with pantoprazole and some with ranitidine. A total of 85.7% of patients with positive results for Acinetobacter and 75.9% for Pseudomonas aeruginosa had taken pantoprazole, while only 14.3% and 24.1% positive cultures for Acinetobacter and Pseudomonas aeruginosa had taken ranitidine, respectively. Therefore, there was a statistically significant between two groups (P < 0.001). In this study, there was also a significant difference between Staphylococcus aureus and Proteus organisms found in cultures of ranitidine and pantoprazole consumer (P < 0.001). This means that, 69.2% and 82.6% of positive cultures for Staphylococcus aureus and Proteus were related to ranitidine respectively, whereas 30.8% and 17.4% of positive cultures for Staphylococcus aureus and Proteus were related to pantoprazole consumers, respectively. Totally, the results between the two groups for each four pathogen were significantly different (P < 0.001) (Table 3).
| Organisms | Drugs | P- Value | |
|---|---|---|---|
| Pantoprazole (%) | Ranitidine (%) | ||
| Acinetobacter | 12 (85.7) | 2 (14.3) | < 0.001 |
| Pseudomonas aeruginosa | 22 (75.9) | 7 (24.1) | |
| Staphylococcus aureus | 4 (30.8) | 9 (69.2) | |
| Proteus | 4 (17.4) | 19 (82.6) | |
| Klebsiella pneumoniae | 6 (42.9) | 8 (57.1) | |
| Others | 1 (14.3) | 6 (85.7) | |
5. Discussion
Multiple factors such as central nervous system diseases, level of consciousness and position of patients, along with ventilator circuit and increase in bacterial bioburden of the upper respiratory and orogastric tracts prepare the condition to establish nosocomial pneumonia. Among the factors that have an effect on the bacterial bioburden of the upper respiratory and orogastric tracts, the role of hospital stay duration, prolonged antibiotic exposures, and gastric acid suppression are prominent (4, 11-15). Interventions and strategies to eradicate oropharyngeal and/or intestinal microbial colonization, such as chlorhexidine oral care, prophylactic aerosolization of antimicrobials, selective decontamination of the digestive tract (SDD), use of sucralfate rather than H2 antagonists for stress ulcer prophylaxis, enteral feeding strategies that preserve gastric pH and also semirecumbent positioning, head elevation, or continuous subglottic suctioning have been frequently used to decrease the incidence of VAP (28-32).
In contrast to a few studies that claimed that routine prophylaxis for stress-related bleeding, even in high-risk patients, seems to not be justified (18), several assessments in high-risk patients (ventilated for > 48 hrs. and coagulopathic) demonstrated that the risk of bleeding outweighs the risk of VAP from pH-modifying agents (29, 33, 34). There are various studies done to assess different gastrointestinal prophylactic agents in their ability to prevent gastrointestinal bleeding and their effect on establishing pneumonia in critically ill patients on mechanical ventilation. According to some of them, sucralfate versus other forms is accompanied by lower risk of pneumonia but may be the higher rates of bleeding (16, 35); one study with small sample size in children argues against this statement (36). A study that has been published by Huang et al., showed that H2-receptor antagonists resulted in no differential effectiveness in treating overt bleeding, however, it had higher rates of gastric colonization and ventilator-associated pneumonia (17). Another study by Kantorova et al. claimed that critically ill mechanical ventilated patients who have received ranitidine had a significantly lower rate of clinically important gastrointestinal bleeding than those treated with sucralfate (18). Although the analyses above, two studies have shown that prophylaxis with sucralfate or histamine-2 receptor antagonists is associated with minimal risk of VAP (37, 38). Two groups that examined the effect of PPIs on nosocomial pneumonia found no significant differences in pneumonia rates compared to histamine-2 receptor antagonists or sucralfate (18, 39). Solouki et al. conducted a study for comparing omeprazole and ranitidine as SRMD prophylaxis, in VAP incidence among ICU patients, and concluded that incidence of VAP did not differ significantly between them (22).
There were few studies that have been done to evaluate pantoprazole (a now widely used SRMD prophylaxis agent for critically ill patients in ICU) and ranitidine (an H2-receptor blocker that is also widely used in these patients) with perspective of GI bleeding, hospital stay days, and VAP development. This issue was investigated by the authors of this article during the time. With this regard, in a study which has been done by Rahimi Bashar et al., the effect of ranitidine and pantoprazole on gastrointestinal bleeding prophylaxis in critically ill patients has been compared and showed that intermittent IV pantoprazole more effectively controls gastric pH and may prevent UGIB in them (23). In another study by Hajiesmaeili et al., it has been shown that despite a higher predicted mortality of critically ill patients in the pantoprazole recipients (about 8%), the ranitidine recipients had a higher mortality rate (13.9%) (24). Bashar et al. compared the effect of pantoprazole and ranitidine on VAP incidence has been compared, and the results showed significantly higher VAP occurrence in the pantoprazole group (25). Another study by Miano et al., yielded the same (26).
As mentioned above, up to our knowledge, there is no study for evaluating different organisms obtained from sputum of the patients who had developed VAP, regarding to their gastrointestinal prophylaxis, and for studying whether there is a relationship between pathogens and SRMD prophylaxis agents or not. The difference observed in this study might be due to an inflammatory response arisen by each agent. Concerning this theory, in a study that has been done by Sasaki and colleagues, lanaprazole has been shown as an inhibitor of rhinovirus replication in tracheal epithelial cells, with reducing cytokine production and decrease in ICAM-1 (intercellular adhesion molecule-1) production (40). Also in some studies it has been showed that PPIs have anti-inflammatory effect by means of neutrophil adhesion to endothelial cells (41). Tabeefar et al. suggested that pantoprazole may have anti-inflammatory effects on critically ill patients (42). In spite of these inflammation regulatory effects of pantoprazole, the more growth of some organisms may be due to constant lower pH, which has been made by pantoprazole and results in bacterial overgrowth and consequent bacterial translocation (43).
Regarding previous studies by authors of this investigation and other researchers, it can be suggested that each bacterium has a unique ability and propensity to grow in specific gastric pH or other systemic changes made by various agents used for SRMD prophylaxis; consequently different prognosis and outcome can be expected by using each agent. Due to lacking sufficient data for confirming this theory, more studies should be done for evaluating patients with ventilator associated pneumonia based on the responsible organisms and regarding to gastrointestinal prophylaxis they were on.
5.1. Conclusion
As mentioned before, according to the results of this study, suggesting the fact that different bacteria have various propensities to grow in specific gastric pH or other systemic changes made by different gastric acid suppressors seems justified; however, due to the small sample size, which confines clinical judgment and external validity, more studies, especially clinical trials, should be done to evaluate more critically ill patients receiving SRMD prophylaxis, with perspective of VAP incidence, the responsible organisms, hospital and ICU stay days, and mortality rate in order to prevent horrid outcomes caused by specific organisms.