Abstract
Background:
Spondylitis is an important osteoarticular manifestation of brucellosis that leads to serious complications.Objectives:
We aimed to investigate various aspects of spondylitis in brucellosis patients in Kermanshah, a highly endemic area in the West of Iran.Methods:
This retrospective, single-center, cross-sectional study investigated 289 brucellosis patients among whom, 32 patients were confirmed to have brucellar spondylitis. The diagnosis of brucellosis was made by Wright or Coombs Wright tests (titers ≥ 1/80) or 2ME test (titer ≥ 1/40). Brucellar spondylitis was confirmed by vertebral MRI or whole-body bone scan. All analyses were done using SPSS 21 software. The chi-square or Fisher exact test was used for assessing associations. P values of < 0.05 were considered statistically significant.Results:
Among 289 patients studied, 32 (11.07%) had spondylitis with a mean age of 53.44 ± 16.06 years. Unpasteurized dairy product consumption, rural residence, and livestock-related occupation were reported by 30 (93.7%), 22 (68.7%), and 28 (87.5%) patients, respectively. Back pain (100%) was the most common symptom while the temperature of ≥ 37.7 (50%) and vertebral column tenderness (50%) were the most observed signs. Brucellar spondylitis was statistically related to age > 40 years, admission duration > 10 days, and ESR > 40 mm/h but not to sex, fever, anemia, and Wright titer. The lumbar disc involvement was the most common involvement (90.6%) in brucellar spondylitis patients. Vertebral body involvement, abnormal marrow signal, and bone marrow edema were observed in all 31 patients diagnosed with MRI.Conclusions:
Brucellar spondylitis should be considered in patients with lower back pain and fever in endemic areas. Positive Wright serology, vertebral body involvement in MRI, and elevated ESR greatly favor the diagnosis of this complication.Keywords
1. Background
As a common zoonotic disease, brucellosis infects 500,000 individuals each year worldwide. It is mostly detected in the Middle East, specifically Iran as an endemic area (1, 2). However, the World Health Organization (WHO) believes that this number is most probably much higher than estimated (3). Kermanshah Province, with the highest rate of infection in Iran, shows an annual incidence of 276.41 per 100,000 population (4). Transmission occurs mainly through consuming unpasteurized dairy products or direct contact with infected animals or their placentas/aborted fetuses (5). Brucellosis is a systemic disease that involves almost all organs and causes clinical complications of various natures (6). However, it most commonly affects the osteoarticular system as observed in over half of the patients (7, 8). The musculoskeletal exhibitions include arthritis, spondylitis, spondylodiscitis, osteomyelitis, tenosynovitis, and bursitis. Among them, spondylitis and spondylodiscitis are predominant and affect lumbar and thoracic vertebra (9). The average age of spondylitis onset has been reported to be 40 years with no sex pattern (10, 11). In terms of age, however, peripheral arthritis and sacroiliitis are observed more commonly at ages less than 45 (12).
The prevalence of brucellar spondylitis has been reported to be 2% - 60% (13). Back pain is a distinct yet not exclusive symptom of this complication that makes the early diagnosis difficult. On the other hand, it is vital to distinguish between the disease and simple back pain as early as possible to prevent its severe neurologic complications such as spinal cord compression (14).
2. Objectives
The present study focused on the frequency of brucellar spondylitis and its clinical and paraclinical manifestations in Kermanshah, Iran.
3. Methods
This retrospective study was performed on 289 patients diagnosed with brucellosis hospitalized in Imam Reza University Hospital of Kermanshah between 2011 and 2016. It is noteworthy to mention that the study protocol completely complied with the principles of the Declaration of Helsinki and the project was affirmed by the Ethics Committee of Kermanshah University of Medical Sciences. Also, a consent form was obtained from the patients’ files, which was voluntarily signed by participants granting permission to anonymously use their data for future scientific purposes. The inclusion criteria were as follows: (A) Confirmed brucellosis by positive Wright or Coombs Wright test (titer ≥ 1/80) or positive 2ME test (titer ≥ 1/40); (B) any evidence of spondylitis in magnetic resonance imaging (MRI) including disc destruction, new bone formation in the vertebral column, intervertebral disc involvement, and paravertebral abscess; and (C) the increased uptake of technetium-99m methylene diphosphonate (99mTc-MDP) in bone scan. Moreover, we obtained demographic data including age, sex, occupation, rural/urban residence, and dairy product consumption. We extracted clinical signs (temperature ≥ 37.7ºC, vertebral tenderness, hepatomegaly, splenomegaly, arthritis, and motor weakness/paralysis) and symptoms (back pain, chills, fever, weakness, myalgia, and sweating) from the patients’ files. Laboratory and imaging data were gathered from the patients’ files, as well. Treatment choices were adopted from the National Guideline for Brucellosis Control and included two types of bi-antibiotic and triple antibiotic. In the bi-antibiotic group, patients received rifampin/co-trimoxazole (480/2400 mg daily). In the triple antibiotic group, patients had three different combinations including: (1) rifampin (600 mg/d) plus doxycycline (200 mg/d) plus gentamicin (240 mg/d); (2) rifampin (600 mg/d) plus doxycycline (200 mg/d) plus streptomycin (1 gr/d); and (3) rifampin (600 mg/d) plus doxycycline (200 mg/d) plus ciprofloxacin (1 g/d). Data were first imported to Excel software and finally, all analyses were done using SPSS 21 software (SPSS Inc., IL, USA). In this regard, mean and standard deviation were used for quantitative data while frequency and percentages were used for qualitative variables. To evaluate the associations, the chi-square or Fisher exact test was used. P values of < 0.05 were considered statistically significant.
4. Results
Among 289 patients studied, 62 (21.4%) were diagnosed with osteoarticular involvement including spondylitis, peripheral arthritis, and sacroiliitis with a frequency of 51.6% (32/62), 25.8% (16/62), and 22.5% (14/62), respectively.
4.1. Demographic, Clinical Manifestations, and Laboratory Findings of Brucellar Spondylitis Patients
Among 32 (11.07%) patients who met the criteria for spondylitis, 12 (37.5%) were females and 20 (62.5%) were males. The age range was 18 to 77 years with a mean of 53.44 ± 16.06 years. The history of unpasteurized dairy product consumption was mentioned by 30 (93.7%) patients. Also, 22 (68.7%) cases were from rural areas while 10 (31.3%) were from urban areas. Moreover, 28 (87.5%) patients declared livestock-related occupation (Table 1).
Demographic, Clinical Manifestations, and Laboratory Findings of 32 Brucellar Spondylitis Patients
| Characteristics | Brucellar Spondylitisa | Range (Min - Max) |
|---|---|---|
| WBC, /µL | 7784.4 ± 2046.09 | (5 - 12.8)*1000 |
| Hemoglobin, g/dL | 12.4 ± 1.74 | (9.7 - 15.9) |
| Platelet, /µL | 302375 ± 71355.42 | (182 - 486)*1000 |
| ESR, mm/h | 53.9 ± 31.50 | (9 - 115) |
| Wright titer | 1:415 ± 1:408.6 | (1:80 - 1:1280) |
| 2ME titer | 1:90 ± 1:189.1 | (1:40 - 1:640) |
| Coombs Wright titer | 1:452 ± 1:290.5 | (1:20 - 1:1280) |
| Age, y | NA | |
| > 40 | 28 (87.5) | |
| ≤ 40 | 4 (12.5) | |
| Sex | NA | |
| Male | 20 (62.5) | |
| Female | 12 (37.5) | |
| Dairy products consumption | NA | |
| Yes | 30 (93.7) | |
| No | 2 (6.3) | |
| Rural residence | NA | |
| Yes | 22 (68.7) | |
| No | 10 (31.3) | |
| Livestock-related occupation | NA | |
| Yes | 28 (87.5) | |
| No | 4 (12.5) | |
| Treatment | NA | |
| Triple antibiotic therapy | 26 (81.3) | |
| Bi-antibiotic therapy | 6 (18.7) |
It is noteworthy to mention that all patients were symptomatic. Accordingly, the most common symptoms of the disease were identified to be back pain, chills, fever, and weakness recorded by 32 (100%), 27 (84%), 26 (81), and 25 (78%) patients, respectively. Temperature over 37.7ºC, vertebral column tenderness, and hepatomegaly were the most common signs with a frequency of 50% (16 cases), 50% (16 cases), and 22% (7 cases), respectively. One patient also complained of neck pain. Four cases (12.5%) exhibited neurologic symptoms including three cases of radiculopathy and one case of simultaneous radiculopathy and paresis of the lower extremity (Table 1). Interestingly, among 32 cases of spondylitis, 15 had spondylitis alone while 14 showed concomitant spondylitis with following manifestations: neurobrucellosis (one case, 3.1%), arthritis (one case, 3.1%), sacroiliitis (two cases, 6.2%), and liver involvement (11 cases, 34.3%) (Table 1). Regarding the treatment, 26 (81.3%) patients received triple antibiotic therapy while 6 (18.7%) received bi-antibiotic treatment (Table 1).
4.2. Comparison of Studied Variables Between Brucellar Spondylitis and other Brucellosis Patients
As observed in Table 2, spondylitis in brucellosis patients was statistically related to the age of over 40 years (P value < 0.001), administration duration of over 10 days (P value = 0.01), and ESR of higher than 40 mm/h (P value = 0.022). On the other hand, sex, fever, anemia, and Wright titer had no significant relationships with spondylitis in brucellosis patients (Table 2).
Comparison of Studied Variables Between Brucellar Spondylitis and Other Brucellosis Patientsa
| Characteristics | Patients with Spondylitis (N = 32) | Patients Without Spondylitis (N = 257) | P Value |
|---|---|---|---|
| Age > 40 years | 28/32 (81.5) | 122/257 (47.4) | < 0.001 |
| Male sex | 20/32 (62.5) | 151/257 (58.7) | 0.684 |
| T ≥ 37.8ºC | 16/32 (50) | 84/255 (32.9) | 0.056 |
| Admission duration > 10 days | 17/32 (53.1) | 78/257 (30.3) | 0.010 |
| Hemoglobin < 12 g/dL | 13/32 (40.6) | 117/251 (46.6) | 0.522 |
| ESR ≥ 40 mm/h | 20/28 (71.4) | 110/227 (48.4) | 0.022 |
| Wright > 1/320 | 8/32 (25) | 54/257 (21) | 0.0604 |
4.3. Imaging
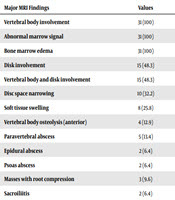
Spondylitis was diagnosed by MRI in 31 patients and the other one patient was diagnosed by bone scan (L4-L5 involvement). Lumbar disc involvement was the most common involvement in brucellar spondylitis patients with a frequency of 90.6% (29 out of 32 patients). Furthermore, L4-L5 was the most involved segment with a frequency of 43.7% (14 patients). Other involvements included lumbosacral and thoracic disc involvement with a frequency of 15.6% (five patients) and 12.5% (four patients), respectively. There was also one (3.1%) patient with cervical spine disc spondylitis (C6-C7). Table 3 shows the MRI findings in 31 patients with brucellar spondylitis. As observed, abscess formation was detected in nine (29%) patients. Furthermore, three (9.6%) patients had evidence of masses with root compression, which might be due to abscess formation (Table 3).
| Major MRI Findings | Values |
|---|---|
| Vertebral body involvement | 31 (100) |
| Abnormal marrow signal | 31 (100) |
| Bone marrow edema | 31 (100) |
| Disk involvement | 15 (48.3) |
| Vertebral body and disk involvement | 15 (48.3) |
| Disc space narrowing | 10 (32.2) |
| Soft tissue swelling | 8 (25.8) |
| Vertebral body osteolysis (anterior) | 4 (12.9) |
| Paravertebral abscess | 5 (13.4) |
| Epidural abscess | 2 (6.4) |
| Psoas abscess | 2 (6.4) |
| Masses with root compression | 3 (9.6) |
| Sacroiliitis | 2 (6.4) |
5. Discussion
Brucellosis is a systemic bacterial disease transmitted from animals to humans, which involves many organs and tissues. Osteoarticular involvements are the most frequent complications associated with brucellosis among which the diagnosis of brucellar spondylitis is often challenging. The mentioned issue can be attributed to clinical presentations of spondylitis often overlapping with many other conditions, which increases the risk of misdiagnosis. Therefore, it seems crucial to approach this manifestation of brucellosis more thoroughly. The frequency of osteoarticular involvement was 21.4% in our study. In similar studies, the rate has been reported as 9% in India (15), 23% in China (16), 46% in rural Uganda (17), 63% in Russia (18) and to top it all, 69% in Anatolia (12). However, our data are more compatible with the results presented by Hashemi et al. (26.8%) from Hamadan (19), the neighboring province of Kermanshah. Among 289 patients included herein, 32 cases had spondylitis giving an overall frequency of 11.07%. This value is very much similar to 11.9% presented by Ebrahimpour et al. from Babol, Iran (20). In other studies, this value ranges from 10% to 45%, which also includes our findings (12, 16, 21-24).
The mean age of our patients was 53 ± 16.06 years (18 to 77 years). There was a statistically significant relationship between older ages and brucellar spondylitis, as 87.5% of our patients aged over 40 years. According to Koubaa et al.’s study, the mean age of patients with spondylitis was 51 years (19 - 74 years) and these patients were older than patients with other manifestations (21). In a similar study, Aktug-Demir et al. (25) showed that the mean age of spondylitis patients was 43 years, which was higher than the age of other cases. These data are also consistent with our results and demonstrate that older age could be a risk factor for brucellar spondylitis.
Sex distribution of the disease was not uniform in our study, as 62.5% of patients were males and 37.5% were females. The ratio was mostly in favor of the male gender in other studies, such as Yang et al.’s study in which 81% of brucellar cases were males (26). Similar to other studies, we found no statistically significant relationship between sex and spondylitis manifestations (20, 23, 27).
In our study, 68.7% of the patients were from rural areas. The history of consuming unpasteurized dairy products and contact with livestock was positive for 93.7% and 87.5% of the patients, respectively. In Solera et al.’s study, rural residence and history of consuming unpasteurized dairy products were considered as brucellosis risk factors (27). In Gokhale et al.’s study, consuming such products (cheese made from raw milk) and contact with livestock were mentioned as the transmission routes of spondylitis (28).
In the present study, back pain (100%), fever (81%), chills (84%), and weakness (78%) were the most common symptoms, similar to the results presented by Koubaa et al. that pointed to back pain, fever, and sweating (21). Persisting back pain (29), neck pain, high fever (13), sweating, and lower limb weakness (14) were also mentioned in case reports.
Patients with spondylitis had a higher mean duration of hospitalization than other patients (12.5 days versus 9.18 days), which was statistically significant. Spondylitis, as a focal impression of brucellosis, requires longer treatment and responds poorly to treatment, leading to a longer period of hospitalization in these cases. On the other hand, the high mean duration of hospitalization is associated with the risk of many health problems such as hospital-acquired infections.
Anemia was recorded in 40.6% of our cases but no leukopenia or thrombocytopenia was observed. In Ioannou et al.’s study, anemia was detected in 55% (30). Koubaa et al. found the prevalence of anemia and leukopenia as 25% and 9.4%, respectively, but no thrombocytopenia was observed (21). The prevalence of leukopenia and thrombocytopenia was 3% and 11%, respectively, in the Solera et al.’s study on 35 patients (27). Similar to these two studies and regardless of the seemingly high prevalence of anemia in our spondylitis patients, no statistically significant relationship was found between this factor and spondylitis manifesting. However, the chronic trend of this pathology could be blamed regarding anemia in patients.
Our study included brucellosis patients affirmed by the positive Wright test. Although the conclusive diagnostic test for brucellosis requires the isolation of the microorganism in cultures as blood culture sensitivity is up to 85% (31), serologic tests are still considered important tools of diagnosis. However, seronegative patients with suspicious manifestations in endemic areas still require blood cultures (32). Interestingly, the prevalence of seronegative cases of brucellosis has been reported as 1% - 2% among patients with osteoarticular manifestations which, as already asserted, needs a blood culture (33). Accordingly, based on our results and data from other studies, routine laboratory tests could not solely be used as a diagnostic tool for spondylitis.
The ESR level was significantly higher in our spondylitis patients than in other cases. The mean ESR level was calculated as 53 mm/h (ranging from 9 to 115 mm/h) and 71.8% had an ESR of higher than 40 mm/h. Likewise, Solera et al. (27) in Spain and Yilmaz et al. (34) in Turkey reported the mean ESR levels of 50 and 48 mm/h, respectively, for their patients. Consistent with our data, these studies also found a statistically significant relationship between high ESR and spondylitis (27, 34). Also, Aktug-Demir et al. believe that, like older age, higher ESR is positively associated with brucellar spondylitis (25). Accordingly, although routine laboratory tests are not of great value to spondylitis diagnosis, high ESR has been observed in most cases and it can be an indicator of response to treatment.
According to the data, MRI is the diagnostic method of choice for brucellosis-induced spondylitis (35, 36). In the current study, the diagnosis was achieved by MRI and bone scan for 31 and one patients, respectively. Bone scan, as a highly sensitive, low specific diagnostic tool, may help with the diagnosis of spondylitis by showing the higher absorption in endplates (37, 38). It is also a rather specific diagnostic technique for osteomyelitis, discitis, and aseptic spinal diseases (39).
Regarding the leading MRI manifestations of spondylitis, lumbar involvement with the prevalence of 90.6% was the most involved area of brucellosis spondylitis in the spine. Interestingly, L4-L5 showed to be the most common affected sites in the lumbar section. The isolated lumbar involvement had a prevalence of 69% and lumbosacral and thoracic involvements were observed in 15.6% and 12.5% of all cases, respectively. Also, only was one patient diagnosed with cervical changes in C6-C7. Similarly, other studies declared the lumbar vertebrae as the most commonly involved site in brucellosis spondylitis (40, 41).
Spinal epidural abscess and soft tissue edema may cause a radiating pain (sciatic pain) to the limb. In our study, four patients had sciatic pain. In Koubaa et al.’s study, sciatic pain was reported in 46% of cases, which is greater than our value (21). This two-fold difference between the values could be explained by the high prevalence of abscess in Koubaa et al.’s research. Limb paresis following spondylitis could be a result of the epidural pressure, paravertebral abscess, or the destruction of the vertebral body because of the pressure on its nearby nerve. Only one out of 32 (3%) patients showed inferior limb muscle weakness (paresis) whose MRI result showed signs of a big epidural abscess pressuring its nearby nerve. Based on these findings, this patient underwent surgery along with antibacterial treatment. Koubaa et al. (21) and Colmenero et al’s studies (22) showed that 12.5% and 8.3% of the patients developed paresis, respectively. The low prevalence of paresis in our study could be related to the early diagnosis and lower prevalence of abscess. Moreover, 50% of our patients were febrile when admitted. This value was reported as 54% in Bodur et al.’s study (23) and 87.5% in Koubaa et al.’s study (21). Vertebral tenderness, as a sign of inflammatory back pain, was detected in 50% of our patients, which is lower than 81% in Colmenero et al’s study (22). Spondylitis involving the vertebral body or paravertebral space involvement could lead to tenderness that, along with lower back pain, is an important clinical feature of brucellar spondylitis (59% of our patients had lower back pain complains). Therefore, it could promote early diagnosis.
In this study, only one patient (3.1%) had the indication for surgical intervention, which is substantially lower than the results by Colmenero et al. that reported 34.4% surgical interventions due to indications such as neurological defects and abscess (41). According to the results, seven (21.8%) cases had abscess among which, five were epidural and the other two were paravertebral abscesses. Of these, two abscesses induced neurologic signs and symptoms as a result of spinal cord compression. The mentioned result was similar to data provided by Bodur et al. (23) and Gokhale et al. (28). However, in another study by Koubaa et al., paravertebral and epidural abscesses had a prevalence of 66% and 59%, respectively (21). Among all the patients, two (10%) cases were diagnosed with psoas abscess, which seems close to the results by Yang et al. with 13% prevalence (26). Although psoas abscess is a rare complication following brucellosis, detecting this pathology does not contradict brucellosis diagnosis (42, 43). As mentioned in the results, the prevalence of concomitant sacroiliitis was 6% in this study, which was lower than the value in other studies reporting the prevalence of 23.7% and 14% (25, 27).
Vertebral body involvement, abnormal marrow signal, and bone marrow edema were certain MRI findings among our brucellar spondylitis patients that together with disk involvement, disc space narrowing, soft tissue swelling, and vertebral body osteolysis could be acceptable MRI features for the diagnosis of brucellar spondylitis. It is noteworthy to mention that a certain number of brucellosis cases are outpatients and therefore, their data are not usually included in such studies as ours. Thus, this could be a limitation to obtaining the precise percentage of spondylitis cases among total brucellar patients.
5.1. Conclusions
Since brucellar spondylitis overlap with other diseases in many clinical manifestations, any sing of lower back pain and fever in brucellosis endemic regions should be investigated for the possibility of this infection. To help with better diagnosis, practitioners and specialists could look for positive Wright serology, vertebral body involvement in MRI, and elevated ESR.
Brucellar spondylitis, being strongly associated with older ages, could lead to severe complications such as paravertebral and epidural abscess and cord compression, resulting in disability, a prolonged course of the disease, and admission duration.
Acknowledgements
References
-
1.
Leylabadlo HE, Bialvaei AZ, Samadi Kafil H. Brucellosis in Iran: Why not eradicated? Clin Infect Dis. 2015;61(10):1629-30. [PubMed ID: 26261203]. https://doi.org/10.1093/cid/civ646.
-
2.
Pakzad R, Pakzad I, Safiri S, Shirzadi MR, Mohammadpour M, Behroozi A, et al. Spatiotemporal analysis of brucellosis incidence in Iran from 2011 to 2014 using GIS. Int J Infect Dis. 2018;67:129-36. [PubMed ID: 29122689]. https://doi.org/10.1016/j.ijid.2017.10.017.
-
3.
Zhang Y, Zhang Q, Zhao CS. Cervical brucellar spondylitis causing incomplete limb paralysis. Rev Soc Bras Med Trop. 2019;52. e20180243. [PubMed ID: 30994799]. https://doi.org/10.1590/0037-8682-0243-2018.
-
4.
Mirnejad R, Jazi FM, Mostafaei S, Sedighi M. Epidemiology of brucellosis in Iran: A comprehensive systematic review and meta-analysis study. Microb Pathog. 2017;109:239-47. [PubMed ID: 28602839]. https://doi.org/10.1016/j.micpath.2017.06.005.
-
5.
Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: A systematic review of disease frequency. PLoS Negl Trop Dis. 2012;6(10). e1865. [PubMed ID: 23145195]. [PubMed Central ID: PMC3493380]. https://doi.org/10.1371/journal.pntd.0001865.
-
6.
Guler S, Kokoglu OF, Ucmak H, Gul M, Ozden S, Ozkan F. Human brucellosis in Turkey: Different clinical presentations. J Infect Dev Ctries. 2014;8(5):581-8. [PubMed ID: 24820461]. https://doi.org/10.3855/jidc.3510.
-
7.
Wang X, Yan Y, Wu F, Su G, Li S, Yuan X, et al. Sixteen Chinese pediatric brucellosis patients onset of fever in non-epidemic areas and 8 developed with osteoarticular involvement. Clin Rheumatol. 2018;37(1):145-9. [PubMed ID: 28924723]. https://doi.org/10.1007/s10067-017-3819-y.
-
8.
Bosilkovski M, Krteva L, Dimzova M, Vidinic I, Sopova Z, Spasovska K. Human brucellosis in Macedonia - 10 years of clinical experience in endemic region. Croat Med J. 2010;51(4):327-36. [PubMed ID: 20718086]. [PubMed Central ID: PMC2931438]. https://doi.org/10.3325/cmj.2010.51.327.
-
9.
Resorlu H, Sacar S, Inceer BS, Akbal A, Gokmen F, Zateri C, et al. Cervical spondylitis and epidural abscess caused by brucellosis: A case report and literature review. Folia Med (Plovdiv). 2016;58(4):289-92. [PubMed ID: 28068278]. https://doi.org/10.1515/folmed-2016-0035.
-
10.
al-Shahed MS, Sharif HS, Haddad MC, Aabed MY, Sammak BM, Mutairi MA. Imaging features of musculoskeletal brucellosis. Radiographics. 1994;14(2):333-48. [PubMed ID: 8190957]. https://doi.org/10.1148/radiographics.14.2.8190957.
-
11.
Mehanic S, Baljic R, Mulabdic V, Huric-Jusufi I, Pinjo F, Topalovic-Cetkovic J, et al. Osteoarticular manifestations of brucellosis. Med Arch. 2012;66(3 Suppl 1):24-6. [PubMed ID: 22937686]. https://doi.org/10.5455/medarh.2012.66.s24-s26.
-
12.
Geyik MF, Gur A, Nas K, Cevik R, Sarac J, Dikici B, et al. Musculoskeletal involvement of brucellosis in different age groups: A study of 195 cases. Swiss Med Wkly. 2002;132(7-8):98-105. [PubMed ID: 11971204].
-
13.
Lee HJ, Hur JW, Lee JW, Lee SR. Brucellar spondylitis. J Korean Neurosurg Soc. 2008;44(4):277-9. [PubMed ID: 19096693]. [PubMed Central ID: PMC2588316]. https://doi.org/10.3340/jkns.2008.44.4.277.
-
14.
Tur BS, Suldur N, Ataman S, Ozturk EA, Bingol A, Atay MB. Brucellar spondylitis: A rare cause of spinal cord compression. Spinal Cord. 2004;42(5):321-4. [PubMed ID: 15123999]. https://doi.org/10.1038/sj.sc.3101571.
-
15.
Mani SSR, Gunasekaran K, Iyyadurai R, Prakash JAJ, Veeraraghavan B, Mishra AK, et al. Clinical spectrum, susceptibility profile, treatment and outcome of culture-confirmed brucellosis from South India. Indian J Med Microbiol. 2018;36(2):289-92. [PubMed ID: 30084427]. https://doi.org/10.4103/ijmm.IJMM_18_236.
-
16.
Jia B, Zhang F, Lu Y, Zhang W, Li J, Zhang Y, et al. The clinical features of 590 patients with brucellosis in Xinjiang, China with the emphasis on the treatment of complications. PLoS Negl Trop Dis. 2017;11(5). e0005577. [PubMed ID: 28459811]. [PubMed Central ID: PMC5426775]. https://doi.org/10.1371/journal.pntd.0005577.
-
17.
Dieckhaus KD, Kyebambe PS. Human brucellosis in rural Uganda: Clinical manifestations, diagnosis, and comorbidities at Kabale Regional Referral Hospital, Kabale, Uganda. Open Forum Infect Dis. 2017;4(4):ofx237. [PubMed ID: 29255733]. [PubMed Central ID: PMC5726460]. https://doi.org/10.1093/ofid/ofx237.
-
18.
Sannikova IV, Makhinya OV, Maleev VV, Deineka DA, Golub OG, Kovalchuk IV, et al. [Brucellosis in the stavropol territory: Results of 15-year follow-up of epidemiological and clinical features]. Ter Arkh. 2015;87(11):11-7. Russian. [PubMed ID: 26821410]. https://doi.org/10.17116/terarkh2015871111-17.
-
19.
Hashemi SH, Keramat F, Ranjbar M, Mamani M, Farzam A, Jamal-Omidi S. Osteoarticular complications of brucellosis in Hamedan, an endemic area in the west of Iran. Int J Infect Dis. 2007;11(6):496-500. [PubMed ID: 17344084]. https://doi.org/10.1016/j.ijid.2007.01.008.
-
20.
Ebrahimpour S, Bayani M, Moulana Z, Hasanjani Roushan MR. Skeletal complications of brucellosis: A study of 464 cases in Babol, Iran. Caspian J Intern Med. 2017;8(1):44-8. [PubMed ID: 28503282]. [PubMed Central ID: PMC5412248].
-
21.
Koubaa M, Maaloul I, Marrakchi C, Lahiani D, Hammami B, Mnif Z, et al. Spinal brucellosis in South of Tunisia: Review of 32 cases. Spine J. 2014;14(8):1538-44. [PubMed ID: 24331843]. https://doi.org/10.1016/j.spinee.2013.09.027.
-
22.
Colmenero JD, Ruiz-Mesa JD, Plata A, Bermudez P, Martin-Rico P, Queipo-Ortuno MI, et al. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin Infect Dis. 2008;46(3):426-33. [PubMed ID: 18181740]. https://doi.org/10.1086/525266.
-
23.
Bodur H, Erbay A, Colpan A, Akinci E. Brucellar spondylitis. Rheumatol Int. 2004;24(4):221-6. [PubMed ID: 12879273]. https://doi.org/10.1007/s00296-003-0350-z.
-
24.
Gonzalez-Gay MA, Garcia-Porrua C, Ibanez D, Garcia-Pais MJ. Osteoarticular complications of brucellosis in an Atlantic area of Spain. J Rheumatol. 1999;26(1):141-5. [PubMed ID: 9918255].
-
25.
Aktug-Demir N, Kolgelier S, Ozcimen S, Sumer S, Demir LS, Inkaya AC. Diagnostic clues for spondylitis in acute brucellosis. Saudi Med J. 2014;35(8):816-20. [PubMed ID: 25129179].
-
26.
Yang B, Hu H, Chen J, He X, Li H. The evaluation of the clinical, laboratory, and radiological findings of 16 cases of Brucellar spondylitis. Biomed Res Int. 2016;2016:8903635. [PubMed ID: 27672661]. [PubMed Central ID: PMC5031813]. https://doi.org/10.1155/2016/8903635.
-
27.
Solera J, Lozano E, Martinez-Alfaro E, Espinosa A, Castillejos ML, Abad L. Brucellar spondylitis: review of 35 cases and literature survey. Clin Infect Dis. 1999;29(6):1440-9. [PubMed ID: 10585793]. https://doi.org/10.1086/313524.
-
28.
Gokhale YA, Ambardekar AG, Bhasin A, Patil M, Tillu A, Kamath J. Brucella spondylitis and sacroiliitis in the general population in Mumbai. J Assoc Physicians India. 2003;51:659-66. [PubMed ID: 14621032].
-
29.
Kilic T, Ozer AF, Ozgen S, Pamir MN. Brucellar spondylitis mimicking lumbar disc herniation. Case report. Paraplegia. 1995;33(3):167-9. [PubMed ID: 7784122]. https://doi.org/10.1038/sc.1995.37.
-
30.
Ioannou S, Karadima D, Pneumaticos S, Athanasiou H, Pontikis J, Zormpala A, et al. Efficacy of prolonged antimicrobial chemotherapy for brucellar spondylodiscitis. Clin Microbiol Infect. 2011;17(5):756-62. [PubMed ID: 20518794]. https://doi.org/10.1111/j.1469-0691.2010.03272.x.
-
31.
Chelli Bouaziz M, Ladeb MF, Chakroun M, Chaabane S. Spinal brucellosis: A review. Skeletal Radiol. 2008;37(9):785-90. [PubMed ID: 17962938]. https://doi.org/10.1007/s00256-007-0371-x.
-
32.
Bozbas GT, Unubol AI, Gurer G. Seronegative brucellosis of the spine: A case of psoas abscess secondary to brucellar spondylitis. Eur J Rheumatol. 2016;3(4):185-7. [PubMed ID: 28149665]. [PubMed Central ID: PMC5283569]. https://doi.org/10.5152/eurjrheum.2015.15082.
-
33.
Janmohammadi N, Roushan MR. False negative serological tests may lead to misdiagnosis and mismanagement in osteoarticular brucellosis. Trop Doct. 2009;39(2):88-90. [PubMed ID: 19299290]. https://doi.org/10.1258/td.2008.080042.
-
34.
Yilmaz E, Parlak M, Akalin H, Heper Y, Ozakin C, Mistik R, et al. Brucellar spondylitis: Review of 25 cases. J Clin Rheumatol. 2004;10(6):300-7. [PubMed ID: 17043537]. https://doi.org/10.1097/01.rhu.0000147048.44396.90.
-
35.
Li T, Li W, Du Y, Gao M, Liu X, Wang G, et al. Discrimination of pyogenic spondylitis from brucellar spondylitis on MRI. Medicine (Baltimore). 2018;97(26). e11195. [PubMed ID: 29952971]. [PubMed Central ID: PMC6039692]. https://doi.org/10.1097/MD.0000000000011195.
-
36.
Bagheri AB, Ahmadi K, Chokan NM, Abbasi B, Akhavan R, Bolvardi E, et al. The diagnostic value of MRI in brucella spondylitis with comparison to clinical and laboratory findings. Acta Inform Med. 2016;24(2):107-10. [PubMed ID: 27147801]. [PubMed Central ID: PMC4851511]. https://doi.org/10.5455/aim.2016.24.107-110.
-
37.
Lim KB, Kwak YG, Kim DY, Kim YS, Kim JA. Back pain secondary to brucella spondylitis in the lumbar region. Ann Rehabil Med. 2012;36(2):282-6. [PubMed ID: 22639756]. [PubMed Central ID: PMC3358688]. https://doi.org/10.5535/arm.2012.36.2.282.
-
38.
Cobbaert K, Pieters A, Devinck M, Devos M, Goethals I, Mielants H. Brucellar spondylodiscitis: Case report. Acta Clin Belg. 2007;62(5):304-7. [PubMed ID: 18229463]. https://doi.org/10.1179/acb.2007.046.
-
39.
Tali ET, Koc AM, Oner AY. Spinal brucellosis. Neuroimaging Clin N Am. 2015;25(2):233-45. [PubMed ID: 25952175]. https://doi.org/10.1016/j.nic.2015.01.004.
-
40.
Chen Y, Yang JS, Li T, Liu P, Liu TJ, He LM, et al. One-stage surgical management for lumbar brucella spondylitis by posterior debridement, autogenous bone graft and instrumentation: A case series of 24 patients. Spine (Phila Pa 1976). 2017;42(19):E1112-8. [PubMed ID: 28157811]. https://doi.org/10.1097/BRS.0000000000002093.
-
41.
Colmenero JD, Reguera JM, Martos F, Sanchez-De-Mora D, Delgado M, Causse M, et al. Complications associated with Brucella melitensis infection: A study of 530 cases. Medicine (Baltimore). 1996;75(4):195-211. [PubMed ID: 8699960]. https://doi.org/10.1097/00005792-199607000-00003.
-
42.
Sharif HS, Aideyan OA, Clark DC, Madkour MM, Aabed MY, Mattsson TA, et al. Brucellar and tuberculous spondylitis: Comparative imaging features. Radiology. 1989;171(2):419-25. [PubMed ID: 2704806]. https://doi.org/10.1148/radiology.171.2.2704806.
-
43.
Smith AS, Weinstein MA, Mizushima A, Coughlin B, Hayden SP, Lakin MM, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol. 1989;153(2):399-405. [PubMed ID: 2750627]. https://doi.org/10.2214/ajr.153.2.399.